Antitumor agents 270. Novel substituted 6-phenyl-4H-furo[3,2-c]pyran-4-one derivatives as potent and highly selective anti-breast cancer agents
- PMID: 20034799
- PMCID: PMC2821697
- DOI: 10.1016/j.bmc.2009.11.049
Antitumor agents 270. Novel substituted 6-phenyl-4H-furo[3,2-c]pyran-4-one derivatives as potent and highly selective anti-breast cancer agents
Abstract
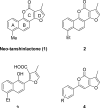
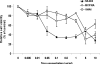
6-Phenyl-4H-furo[3,2-c]pyran-4-one derivatives based on neo-tashinlactone (1) were synthesized and evaluated as novel anti-breast cancer agents. Compounds 10-13, 23, 25, and 27 showed potent inhibition against the SK-BR-3 breast cancer cell line. Importantly, 25 and 27 showed the highest cancer cell line selectivity, being approximately 100-250-fold more potent against SK-BR-3 (ED(50) 0.28 and 0.44microM, respectively) compared with other cancer cell lines tested. In addition, 25 displayed low cytotoxicity against normal breast cell lines 184A1 and MCF10A. Compounds 25 and 27 merit further investigation in our continuing program to generate and develop selective anti-breast cancer agents.
Copyright 2009 Elsevier Ltd. All rights reserved.
Figures

References
-
- Coley HM. Mechanisms and strategies to overcome chemotherapy resistance in metastatic breast cancer. Cancer Treat Rev. 2008;34:378–90. - PubMed
-
- Wang X, Bastow KF, Sun CM, Lin YL, Yu HJ, Don MJ, Wu TS, Nakamura S, Lee KH. Antitumor Agents. 239. Isolation, structure elucidation, total synthesis, and anti-breast cancer activity of neo-tanshinlactone from Salvia miltiorrhiza. J Med Chem. 2004;47:5816–9. - PubMed
-
- Albrand G, Terret C. Early breast cancer in the elderly: assessment and management considerations. Drugs Aging. 2008;25:35–45. - PubMed
-
- Breast Cancer Facts & Figures. 2008.
-
- Chari RVJ. Targeted Cancer Therapy: Conferring Specificity to Cytotoxic Drugs. Acc. Chem. Res. 2008;41:98–107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

