Moderate oxygen augments lipopolysaccharide-induced lung injury in mice
- PMID: 20034961
- PMCID: PMC2838673
- DOI: 10.1152/ajplung.00308.2009
Moderate oxygen augments lipopolysaccharide-induced lung injury in mice
Abstract
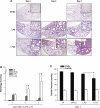
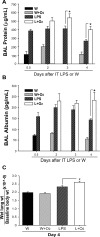
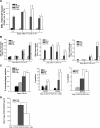
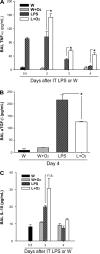
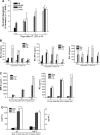
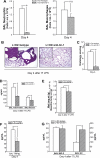
Despite the associated morbidity and mortality, underlying mechanisms leading to the development of acute lung injury (ALI) remain incompletely understood. Frequently, ALI develops in the hospital, coinciding with institution of various therapies, including the use of supplemental oxygen. Although pathological evidence of hyperoxia-induced ALI in humans has yet to be proven, animal studies involving high oxygen concentration reproducibly induce ALI. The potentially injurious role of lower and presumably safer oxygen concentrations has not been well characterized in any species. We hypothesized that in the setting of a preexisting insult to the lung, the addition of moderate-range oxygen can augment lung injury. Our model of low-dose intratracheal LPS (IT LPS) followed by 60% oxygen caused a significant increase in ALI compared with LPS or oxygen alone with increased alveolar neutrophils, histological injury, and epithelial barrier permeability. In the LPS plus oxygen group, regulatory T cell number was reduced, and macrophage activation markers were increased, compared with LPS alone. Antibody-mediated depletion of neutrophils significantly abrogated the observed lung injury for all measured factors. The enhanced presence of alveolar neutrophils in the setting of LPS and oxygen is due, at least in part, to elevated chemokine gradients signaling neutrophils to the alveolar space. We believe these results strongly support an effect of lower concentrations of oxygen to augment the severity of a mild preexisting lung injury and warrants further investigation in both animals and humans.
Figures
References
-
- Altemeier WA, Sinclair SE. Hyperoxia in the intensive care unit: why more is not always better. Curr Opin Crit Care 13: 73–78, 2007 - PubMed
-
- Baleeiro CE, Christensen PJ, Morris SB, Mendez MP, Wilcoxen SE, Paine R., 3rd GM-CSF and the impaired pulmonary innate immune response following hyperoxic stress. Am J Physiol Lung Cell Mol Physiol 291: L1246–L1255, 2006 - PubMed
-
- Baleeiro CE, Wilcoxen SE, Morris SB, Standiford TJ, Paine R Sublethal hyperoxia impairs pulmonary innate immunity. J Immunol 171: 955–963, 2003 - PubMed
-
- Barber RE, Hamilton WK. Oxygen toxicity in man. A prospective study in patients with irreversible brain damage. N Engl J Med 283: 1478–1484, 1970 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

