Loss of nuclear poly(A)-binding protein 1 causes defects in myogenesis and mRNA biogenesis
- PMID: 20035013
- PMCID: PMC2830829
- DOI: 10.1093/hmg/ddp569
Loss of nuclear poly(A)-binding protein 1 causes defects in myogenesis and mRNA biogenesis
Abstract
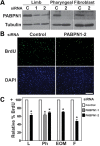
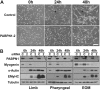
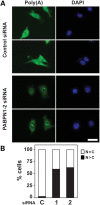
The nuclear poly(A)-binding protein 1 (PABPN1) is a ubiquitously expressed protein that plays a critical role in polyadenylation. Short expansions of the polyalanine tract in the N-terminus of PABPN1 lead to oculopharyngeal muscular dystrophy (OPMD), which is an adult onset disease characterized by eyelid drooping, difficulty in swallowing and weakness in the proximal limb muscles. Although significant data from in vitro biochemical assays define the function of PABPN1 in control of poly(A) tail length, little is known about the role of PABPN1 in mammalian cells. To assess the function of PABPN1 in mammalian cells and specifically in cells affected in OPMD, we examined the effects of PABPN1 depletion using siRNA in primary mouse myoblasts from extraocular, pharyngeal and limb muscles. PABPN1 knockdown significantly decreased cell proliferation and myoblast differentiation during myogenesis in vitro. At the molecular level, PABPN1 depletion in myoblasts led to a shortening of mRNA poly(A) tails, demonstrating the cellular function of PABPN1 in polyadenylation control in a mammalian cell. In addition, PABPN1 depletion caused nuclear accumulation of poly(A) RNA, revealing that PABPN1 is required for proper poly(A) RNA export from the nucleus. Together, these experiments demonstrate that PABPN1 plays an essential role in myoblast proliferation and differentiation, suggesting that it is required for muscle regeneration and maintenance in vivo.
Figures


References
-
- Kuhn U., Wahle E. Structure and function of poly(A) binding proteins. Biochim. Biophys. Acta. 2004;1678:67–84. - PubMed
-
- Kuhn U., Nemeth A., Meyer S., Wahle E. The RNA binding domains of the nuclear poly(A)-binding protein. J. Biol. Chem. 2003;278:16916–16925. - PubMed
-
- Fan X., Dion P., Laganiere J., Brais B., Rouleau G.A. Oligomerization of polyalanine expanded PABPN1 facilitates nuclear protein aggregation that is associated with cell death. Hum. Mol. Genet. 2001;10:2341–2351. - PubMed
-
- Calado A., Tome F.M., Brais B., Rouleau G.A., Kuhn U., Wahle E., Carmo-Fonseca M. Nuclear inclusions in oculopharyngeal muscular dystrophy consist of poly(A) binding protein 2 aggregates which sequester poly(A) RNA. Hum. Mol. Genet. 2000;9:2321–2328. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

