Anti-Fas gene therapy prevents doxorubicin-induced acute cardiotoxicity through mechanisms independent of apoptosis
- PMID: 20035047
- PMCID: PMC2808076
- DOI: 10.2353/ajpath.2010.090222
Anti-Fas gene therapy prevents doxorubicin-induced acute cardiotoxicity through mechanisms independent of apoptosis
Abstract
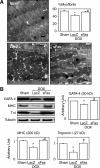
Activation of Fas signaling is a key mediator of doxorubicin cardiotoxicity, which involves both cardiomyocyte apoptosis and myocardial inflammation. In this study, acute cardiotoxicity was induced in mice by doxorubicin, and some mice simultaneously received an intramuscular injection of adenoviral vector encoding mouse soluble Fas (sFas) gene (Ad.CAG-sFas), an inhibitor of Fas/Fas ligand interaction. Two weeks later, left ventricular dilatation and dysfunction were apparent in the LacZ-treated control group, but both were significantly mitigated in the sFas-treated group. The in situ nick-end labeling-positive rate were similar in the two groups, and although electron microscopy revealed cardiomyocyte degeneration, no apoptotic structural features and no activation of caspases were detected, suggesting an insignificant role of apoptosis in this model. Instead, sFas treatment reversed doxorubicin-induced down-regulation of GATA-4 and attenuated ubiquitination of myosin heavy chain and troponin I to preserve these sarcomeric proteins. In addition, doxorubicin-induced significant leukocyte infiltration, fibrosis, and oxidative damage to the myocardium, all of which were largely reversed by sFas treatment. sFas treatment also suppressed doxorubicin-induced p53 overexpression, phosphorylation of c-Jun N-terminal kinase, c-Jun, and inhibitor of nuclear factor-kappaB, as well as production of cyclooxygenase-2 and monocyte chemoattractant protein-1, and it restored extracellular signal-regulated kinase activation. Therefore, sFas gene therapy prevents the progression of doxorubicin-induced acute cardiotoxicity, with accompanying attenuation of the cardiomyocyte degeneration, inflammation, fibrosis, and oxidative damage caused by Fas signaling.
Figures






Similar articles
-
Cardiac-targeted expression of soluble fas attenuates doxorubicin-induced cardiotoxicity in mice.J Pharmacol Exp Ther. 2009 Mar;328(3):740-8. doi: 10.1124/jpet.108.146423. Epub 2008 Dec 9. J Pharmacol Exp Ther. 2009. PMID: 19066339 Free PMC article.
-
Treatment with an adenoviral vector encoding hepatocyte growth factor mitigates established cardiac dysfunction in doxorubicin-induced cardiomyopathy.Am J Physiol Heart Circ Physiol. 2008 Feb;294(2):H1048-57. doi: 10.1152/ajpheart.01102.2007. Epub 2007 Dec 14. Am J Physiol Heart Circ Physiol. 2008. PMID: 18083897
-
Rac1 signalling mediates doxorubicin-induced cardiotoxicity through both reactive oxygen species-dependent and -independent pathways.Cardiovasc Res. 2013 Jan 1;97(1):77-87. doi: 10.1093/cvr/cvs309. Epub 2012 Oct 1. Cardiovasc Res. 2013. PMID: 23027656
-
Protection against lipopolysaccharide-induced myocardial dysfunction in mice by cardiac-specific expression of soluble Fas.J Mol Cell Cardiol. 2008 Jan;44(1):160-9. doi: 10.1016/j.yjmcc.2007.09.016. Epub 2007 Oct 4. J Mol Cell Cardiol. 2008. PMID: 17996250
-
Baicalein alleviates doxorubicin-induced cardiotoxicity via suppression of myocardial oxidative stress and apoptosis in mice.Life Sci. 2016 Jan 1;144:8-18. doi: 10.1016/j.lfs.2015.11.018. Epub 2015 Nov 29. Life Sci. 2016. PMID: 26606860
Cited by
-
ZAK is required for doxorubicin, a novel ribotoxic stressor, to induce SAPK activation and apoptosis in HaCaT cells.Cancer Biol Ther. 2010 Aug 1;10(3):258-66. doi: 10.4161/cbt.10.3.12367. Epub 2010 Aug 13. Cancer Biol Ther. 2010. PMID: 20559024 Free PMC article.
-
Apoptotic cell death in disease-Current understanding of the NCCD 2023.Cell Death Differ. 2023 May;30(5):1097-1154. doi: 10.1038/s41418-023-01153-w. Epub 2023 Apr 26. Cell Death Differ. 2023. PMID: 37100955 Free PMC article. Review.
-
Cardiac gene therapy: are we there yet?Gene Ther. 2016 Aug;23(8-9):635-48. doi: 10.1038/gt.2016.43. Epub 2016 Apr 29. Gene Ther. 2016. PMID: 27128687 Review.
-
Gene Therapy for Cardiovascular Disease: Clinical Perspectives.Yonsei Med J. 2024 Oct;65(10):557-571. doi: 10.3349/ymj.2024.0127. Yonsei Med J. 2024. PMID: 39313446 Free PMC article. Review.
-
Gallic Acid Inhibits Mesaconitine-Activated TRPV1-Channel-Induced Cardiotoxicity.Evid Based Complement Alternat Med. 2022 Apr 13;2022:5731372. doi: 10.1155/2022/5731372. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35463061 Free PMC article.
References
-
- Olson RD, Mushlin P. Doxorubicin cardiotoxicity: analysis of prevailing hypotheses. FASEB J. 1990;4:3076–3086. - PubMed
-
- Shan K, Lincoff AM, Young JB. Anthracycline-induced cardiotoxicity. Ann Intern Med. 1996;125:47–58. - PubMed
-
- Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med. 1998;339:900–905. - PubMed
-
- Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56:185–229. - PubMed
-
- Nagata S. Apoptosis by death factor. Cell. 1997;8:355–365. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

