Remodeling of the fetal collecting duct epithelium
- PMID: 20035053
- PMCID: PMC2808071
- DOI: 10.2353/ajpath.2010.090389
Remodeling of the fetal collecting duct epithelium
Abstract
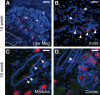
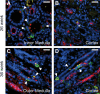
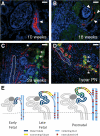
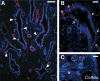
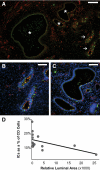
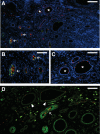
Congenital urinary tract obstruction induces changes to the renal collecting duct epithelium, including alteration and depletion of intercalated cells. To study the effects of obstruction on the ontogeny of intercalated cell development, we examined normal and obstructed human fetal and postnatal kidneys. In the normal human fetal kidney, intercalated cells originated in the medullary collecting duct at 8 weeks gestation and remained most abundant in the inner medulla throughout gestation. In the cortex, intercalated cells were rare at 18 and 26 weeks gestation and observed at low abundance at 36 weeks gestation. Although early intercalated cells exhibit an immature phenotype, Type A intercalated cells predominated in the inner and outer medullae at 26 and 36 weeks gestation with other intercalated cell subtypes observed rarely. Postnatally, the collecting duct epithelium underwent a remodeling whereby intercalated cells become abundant in the cortex yet absent from the inner medulla. In 18-week obstructed kidneys with mild to moderate injury, the intercalated cells became more abundant and differentiated than the equivalent age-matched normal kidney. In contrast, more severely injured ducts of the late obstructed kidney exhibited a significant reduction in intercalated cells. These studies characterize the normal ontogeny of human intercalated cell development and suggest that obstruction induces premature remodeling and differentiation of the fetal collecting duct epithelium.
Figures



Similar articles
-
Collecting duct epithelial-mesenchymal transition in fetal urinary tract obstruction.Kidney Int. 2007 Oct;72(8):936-44. doi: 10.1038/sj.ki.5002457. Epub 2007 Aug 1. Kidney Int. 2007. PMID: 17667982
-
Differentiation of intercalated cells in developing rat kidney: an immunohistochemical study.Am J Physiol. 1994 Jun;266(6 Pt 2):F977-90. doi: 10.1152/ajprenal.1994.266.6.F977. Am J Physiol. 1994. PMID: 8023977
-
Characterization of fetal ovine renal dysplasia after mid-gestation ureteral obstruction.Clin Invest Med. 1996 Dec;19(6):444-52. Clin Invest Med. 1996. PMID: 8959354
-
Mechanisms and regulation of ion transport in the renal collecting duct.Comp Biochem Physiol A Comp Physiol. 1988;90(4):701-7. doi: 10.1016/0300-9629(88)90687-1. Comp Biochem Physiol A Comp Physiol. 1988. PMID: 2460286 Review.
-
Collecting duct intercalated cell function and regulation.Clin J Am Soc Nephrol. 2015 Feb 6;10(2):305-24. doi: 10.2215/CJN.08880914. Epub 2015 Jan 28. Clin J Am Soc Nephrol. 2015. PMID: 25632105 Free PMC article. Review.
Cited by
-
Radiolabeling and in vivo imaging of transplanted renal lineages differentiated from human embryonic stem cells in fetal rhesus monkeys.Mol Imaging Biol. 2012 Apr;14(2):197-204. doi: 10.1007/s11307-011-0487-1. Mol Imaging Biol. 2012. PMID: 21479709 Free PMC article.
-
Genetic Deletion of P2Y2 Receptor Offers Long-Term (5 Months) Protection Against Lithium-Induced Polyuria, Natriuresis, Kaliuresis, and Collecting Duct Remodeling and Cell Proliferation.Front Physiol. 2018 Dec 17;9:1765. doi: 10.3389/fphys.2018.01765. eCollection 2018. Front Physiol. 2018. PMID: 30618788 Free PMC article.
-
Prognostic factors and biomarkers of congenital obstructive nephropathy.Pediatr Nephrol. 2016 Sep;31(9):1411-20. doi: 10.1007/s00467-015-3291-3. Epub 2015 Dec 14. Pediatr Nephrol. 2016. PMID: 26667236 Review.
-
Specification of ion transport cells in the Xenopus larval skin.Development. 2011 Feb;138(4):705-14. doi: 10.1242/dev.055699. Development. 2011. PMID: 21266406 Free PMC article.
-
Pendrin-A New Target for Diuretic Therapy?J Am Soc Nephrol. 2016 Dec;27(12):3499-3501. doi: 10.1681/ASN.2016070720. Epub 2016 Aug 11. J Am Soc Nephrol. 2016. PMID: 27516234 Free PMC article. No abstract available.
References
-
- Anumba DO, Scott JE, Plant ND, Robson SC. Diagnosis and outcome of fetal lower urinary tract obstruction in the northern region of England. Prenat Diagn. 2005;25:7–13. - PubMed
-
- Hayden SA, Russ PD, Pretorius DH, Manco-Johnson ML, Clewell WH. Posterior urethral obstruction. Prenatal sonographic findings and clinical outcome in fourteen cases, J Ultrasound Med. 1988;7:371–375. - PubMed
-
- Nakayama DK, Harrison MR, de Lorimier AA. Prognosis of posterior urethral valves presenting at birth. J Pediatr Surg. 1986;21:43–45. - PubMed
-
- Thomas DF, Irving HC, Arthur RJ. Pre-natal diagnosis: how useful is it? Br J Urol. 1985;57:784–787. - PubMed
-
- Mahony BS, Callen PW, Filly RA. Fetal urethral obstruction: uS evaluation. Radiology. 1985;157:221–224. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

