Persistent hijacking of brain proteasomes in HIV-associated dementia
- PMID: 20035054
- PMCID: PMC2808094
- DOI: 10.2353/ajpath.2010.090390
Persistent hijacking of brain proteasomes in HIV-associated dementia
Abstract
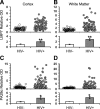
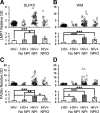
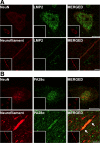
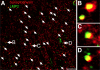
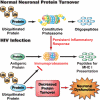
Immunoproteasome induction sustains class 1 antigen presentation and immunological vigilance against HIV-1 in the brain. Investigation of HIV-1-associated alterations in brain protein turnover by the ubiquitin-proteasome system was performed by (1) determining proteasome subunit changes associated with persistent brain inflammation due to HIV-1; (2) determining whether these changes are related to HIV-1 neurocognitive disturbances, encephalitis, and viral loads; and (3) localizing proteasome subunits in brain cells and synapses. On the basis of neurocognitive performance, virological, and immunological measurements obtained within 6 months before death, 153 autopsy cases were selected. Semiquantitative immunoblot analysis performed in the dorsolateral prefrontal cortex revealed up to threefold induction of immunoproteasome subunits LMP7 and PA28alpha in HIV-1-infected subjects and was strongly related to diagnoses of neuropsychological impairment and HIV encephalitis. Low performance on neurocognitive tests specific for dorsolateral prefrontal cortex functioning domains was selectively correlated with immunoproteasome induction. Immunohistochemistry and laser confocal microscopy were then used to localize immunoproteasome subunits to glial and neuronal elements including perikarya, dystrophic axons, and synapses. In addition, HIV loads in brain tissue, cerebrospinal fluid, and blood plasma were robustly correlated to immunoproteasome levels. This persistent "hijacking" of the proteasome by HIV-1-mediated inflammatory response and immunoproteasome induction in the brain is hypothesized to impede turnover of folded proteins in brain cells. This would disrupt neuronal and synaptic protein dynamics, contributing to HIV-1 neurocognitive disturbances.
Figures





Similar articles
-
Proteasomal expression, induction of immunoproteasome subunits, and local MHC class I presentation in myofibrillar myopathy and inclusion body myositis.J Neuropathol Exp Neurol. 2004 May;63(5):484-98. doi: 10.1093/jnen/63.5.484. J Neuropathol Exp Neurol. 2004. PMID: 15198127
-
Synaptic proteins linked to HIV-1 infection and immunoproteasome induction: proteomic analysis of human synaptosomes.J Neuroimmune Pharmacol. 2010 Mar;5(1):92-102. doi: 10.1007/s11481-009-9168-0. Epub 2009 Aug 20. J Neuroimmune Pharmacol. 2010. PMID: 19693676 Free PMC article.
-
Brain aging in acquired immunodeficiency syndrome: increased ubiquitin-protein conjugate is correlated with decreased synaptic protein but not amyloid plaque accumulation.J Neurovirol. 2004 Apr;10(2):98-108. doi: 10.1080/13550280490279816. J Neurovirol. 2004. PMID: 15204928
-
The role of the ubiquitin-proteasome pathway in MHC class I antigen processing: implications for vaccine design.Curr Mol Med. 2001 Dec;1(6):665-76. doi: 10.2174/1566524013363230. Curr Mol Med. 2001. PMID: 11899255 Review.
-
Differential processing of class-I-restricted epitopes by the standard proteasome and the immunoproteasome.Curr Opin Immunol. 2001 Apr;13(2):147-53. doi: 10.1016/s0952-7915(00)00197-7. Curr Opin Immunol. 2001. PMID: 11228406 Review.
Cited by
-
Mechanisms of HIV-1 Tat neurotoxicity via CDK5 translocation and hyper-activation: role in HIV-associated neurocognitive disorders.Curr HIV Res. 2015;13(1):43-54. doi: 10.2174/1570162x13666150311164201. Curr HIV Res. 2015. PMID: 25760044 Free PMC article. Review.
-
Update on HIV dementia and HIV-associated neurocognitive disorders.Curr Neurol Neurosci Rep. 2014 Aug;14(8):468. doi: 10.1007/s11910-014-0468-2. Curr Neurol Neurosci Rep. 2014. PMID: 24938216 Review.
-
Modifications in acute phase and complement systems predict shifts in cognitive status of HIV-infected patients.AIDS. 2017 Jun 19;31(10):1365-1378. doi: 10.1097/QAD.0000000000001503. AIDS. 2017. PMID: 28574961 Free PMC article.
-
A novel role for the immunoproteasome in retinal function.Invest Ophthalmol Vis Sci. 2011 Feb 9;52(2):714-23. doi: 10.1167/iovs.10-6032. Print 2011 Feb. Invest Ophthalmol Vis Sci. 2011. PMID: 20881299 Free PMC article.
-
HIV associated neurocognitive disorders in the modern antiviral treatment era: prevalence, characteristics, biomarkers, and effects of treatment.Curr HIV/AIDS Rep. 2014 Sep;11(3):317-24. doi: 10.1007/s11904-014-0221-0. Curr HIV/AIDS Rep. 2014. PMID: 24966139 Review.
References
-
- McArthur JC, Brew BJ, Nath A. Neurological complications of HIV infection. Lancet Neurol. 2005;4:543–555. - PubMed
-
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. - PubMed
-
- Williams KC, Hickey WF. Central nervous system damage, monocytes and macrophages, and neurological disorders in AIDS. Annu Rev Neurosci. 2002;25:537–562. - PubMed
-
- Budka H. Neuropathology of human immunodeficiency virus infection. Brain Pathol. 1991;1:163–175. - PubMed
-
- Wiley CA, Achim C. Human immunodeficiency virus encephalitis is the pathological correlate of dementia in acquired immunodeficiency syndrome. Ann Neurol. 1994;36:673–676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

