Inhibition of CD36-dependent phagocytosis by prostaglandin E2 contributes to the development of endometriosis
- PMID: 20035060
- PMCID: PMC2808090
- DOI: 10.2353/ajpath.2010.090551
Inhibition of CD36-dependent phagocytosis by prostaglandin E2 contributes to the development of endometriosis
Abstract
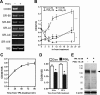
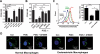
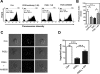
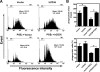
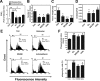
Dysfunction in macrophage-mediated phagocytosis of aberrant cells that undergo retrograde transport to the peritoneal cavity is considered an important factor in the development of endometriosis. However, the mechanisms responsible for the loss of function of macrophages remain largely unknown. Herein, we report that prostaglandin (PG) E(2), via the EP2 receptor-dependent signaling pathway, inhibits the expression of CD36 in peritoneal macrophages, resulting in reduced phagocytic ability. PGE(2)-mediated inhibition of macrophage phagocytic capability was restored by ectopic expression of CD36. Treatment with PGE(2) inhibited CD36-dependent phagocytosis of peritoneal macrophages and increased the number and size of endometriotic lesions in mice. In contrast, blockade of PGE(2) production by cyclooxygenase inhibitors enhanced the phagocytic ability of peritoneal macrophages and reduced endometriotic lesion formation. Taken together, our findings reveal a potential mechanism of immune dysfunction during endometriosis development and may contribute to the design of an effective prevention/treatment regimen.
Figures

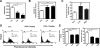
Similar articles
-
Suppression of annexin A2 by prostaglandin E₂ impairs phagocytic ability of peritoneal macrophages in women with endometriosis.Hum Reprod. 2013 Apr;28(4):1045-53. doi: 10.1093/humrep/det003. Epub 2013 Jan 22. Hum Reprod. 2013. PMID: 23340055
-
Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP.J Immunol. 2004 Jul 1;173(1):559-65. doi: 10.4049/jimmunol.173.1.559. J Immunol. 2004. PMID: 15210817
-
Exogenous CXCL4 infusion inhibits macrophage phagocytosis by limiting CD36 signalling to enhance post-myocardial infarction cardiac dilation and mortality.Cardiovasc Res. 2019 Feb 1;115(2):395-408. doi: 10.1093/cvr/cvy211. Cardiovasc Res. 2019. PMID: 30169632 Free PMC article.
-
Role of iron overload-induced macrophage apoptosis in the pathogenesis of peritoneal endometriosis.Reproduction. 2014 Jun;147(6):R199-207. doi: 10.1530/REP-13-0552. Epub 2014 Mar 5. Reproduction. 2014. PMID: 24599836 Review.
-
Regulation and modulation of abnormal immune responses in endometriosis.Ann N Y Acad Sci. 2002 Mar;955:159-73; discussion 199-200, 396-406. doi: 10.1111/j.1749-6632.2002.tb02777.x. Ann N Y Acad Sci. 2002. PMID: 11949945 Review.
Cited by
-
Suppression of COUP-TFII by proinflammatory cytokines contributes to the pathogenesis of endometriosis.J Clin Endocrinol Metab. 2014 Mar;99(3):E427-37. doi: 10.1210/jc.2013-3717. Epub 2014 Jan 1. J Clin Endocrinol Metab. 2014. PMID: 24423359 Free PMC article.
-
Estrogen Secreted by Mesenchymal Stem Cells Necessarily Determines Their Feasibility of Therapeutical Application.Sci Rep. 2015 Oct 19;5:15286. doi: 10.1038/srep15286. Sci Rep. 2015. PMID: 26478095 Free PMC article.
-
Promotion of BST2 expression by the transcription factor IRF6 affects the progression of endometriosis.Front Immunol. 2023 Apr 18;14:1115504. doi: 10.3389/fimmu.2023.1115504. eCollection 2023. Front Immunol. 2023. PMID: 37143676 Free PMC article.
-
Indoleamine 2,3-dioxygenase-1 (IDO1) in human endometrial stromal cells induces macrophage tolerance through interleukin-33 in the progression of endometriosis.Int J Clin Exp Pathol. 2014 May 15;7(6):2743-57. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25031694 Free PMC article.
-
Caulis Sargentodoxae Prescription Plays a Therapeutic Role with Decreased Inflammatory Cytokines in Peritoneal Fluid in the Rat Endometriosis Model.Evid Based Complement Alternat Med. 2020 May 29;2020:9627907. doi: 10.1155/2020/9627907. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 32595753 Free PMC article.
References
-
- Simoens S, Hummelshoj L, D'Hooghe T. Endometriosis: cost estimates and methodological perspective. Hum Reprod Update. 2007;13:395–404. - PubMed
-
- Sampson JA. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422–425. - PubMed
-
- Wu MH, Shoji Y, Chuang PC, Tsai SJ. Endometriosis: disease pathophysiology and the role of prostaglandins. Expert Rev Mol Med. 2007;9:1–20. - PubMed
-
- Ozkan S, Murk W, Arici A. Endometriosis and infertility: epidemiology and evidence-based treatments. Ann NY Acad Sci. 2008;1127:92–100. - PubMed
-
- Dunselman GA, Hendrix MG, Bouckaert PX, Evers JL. Functional aspects of peritoneal macrophages in endometriosis of women. J Reprod Fertil. 1988;82:707–710. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

