The C terminus of cardiac troponin I stabilizes the Ca2+-activated state of tropomyosin on actin filaments
- PMID: 20035081
- PMCID: PMC2834238
- DOI: 10.1161/CIRCRESAHA.109.210047
The C terminus of cardiac troponin I stabilizes the Ca2+-activated state of tropomyosin on actin filaments
Abstract
Rationale: Ca(2+) control of troponin-tropomyosin position on actin regulates cardiac muscle contraction. The inhibitory subunit of troponin, cardiac troponin (cTn)I is primarily responsible for maintaining a tropomyosin conformation that prevents crossbridge cycling. Despite extensive characterization of cTnI, the precise role of its C-terminal domain (residues 193 to 210) is unclear. Mutations within this region are associated with restrictive cardiomyopathy, and C-terminal deletion of cTnI, in some species, has been associated with myocardial stunning.
Objective: We sought to investigate the effect of a cTnI deletion-removal of 17 amino acids from the C terminus- on the structure of troponin-regulated tropomyosin bound to actin.
Methods and results: A truncated form of human cTnI (cTnI(1-192)) was expressed and reconstituted with troponin C and troponin T to form a mutant troponin. Using electron microscopy and 3D image reconstruction, we show that the mutant troponin perturbs the positional equilibrium dynamics of tropomyosin in the presence of Ca(2+). Specifically, it biases tropomyosin position toward an "enhanced C-state" that exposes more of the myosin-binding site on actin than found with wild-type troponin.
Conclusions: In addition to its well-established role of promoting the so-called "blocked-state" or "B-state," cTnI participates in proper stabilization of tropomyosin in the "Ca(2+)-activated state" or "C-state." The last 17 amino acids perform this stabilizing role. The data are consistent with a "fly-casting" model in which the mobile C terminus of cTnI ensures proper conformational switching of troponin-tropomyosin. Loss of actin-sensing function within this domain, by pathological proteolysis or cardiomyopathic mutation, may be sufficient to perturb tropomyosin conformation.
Figures


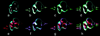

References
-
- Bolli R, Marban E. Molecular and Cellular Mechanisms of Myocardial Stunning. Physiol Rev. 1999;79:609–634. - PubMed
-
- Kimura A, Harada H, Park J-E, Nishi H, Satoh M, Takahashi M, Hiroi S, Sasaoka T, Ohbuchi N, Nakamura T, Koyanagi T, Hwang T-H, Choo J-A, Chung K-S, Hasegawa A, Nagai R, Okazaki O, Nakamura H, Matsuzaki M, Sakamoto T, Toshima H, Koga Y, Imaizumi T, Sasazuki T. Mutations in the cardiac troponin I gene associated with hypertrophic cardiomyopathy. Nat Gen. 1997;16:379–382. - PubMed
-
- Gao WD, Atar D, Liu Y, Perez NG, Murphy AM, Marban E. Role of troponin I proteolysis in the pathogenesis of stunned myocardium. Circ Res. 1997;80:393–399. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 AR41637/AR/NIAMS NIH HHS/United States
- R01 AR34711/AR/NIAMS NIH HHS/United States
- R01 HL63038/HL/NHLBI NIH HHS/United States
- P01 AR041637/AR/NIAMS NIH HHS/United States
- P01 HL077180/HL/NHLBI NIH HHS/United States
- R01 HL36153/HL/NHLBI NIH HHS/United States
- R01 HL092252/HL/NHLBI NIH HHS/United States
- R37 HL036153/HL/NHLBI NIH HHS/United States
- R01 HL063038/HL/NHLBI NIH HHS/United States
- N01 HV028180/HV/NHLBI NIH HHS/United States
- R01 HL036153/HL/NHLBI NIH HHS/United States
- R01 AR034711/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

