Role of mesodermal FGF8 and FGF10 overlaps in the development of the arterial pole of the heart and pharyngeal arch arteries
- PMID: 20035084
- PMCID: PMC2843098
- DOI: 10.1161/CIRCRESAHA.109.201665
Role of mesodermal FGF8 and FGF10 overlaps in the development of the arterial pole of the heart and pharyngeal arch arteries
Abstract
Rationale: The genes encoding fibroblast growth factor (FGF) 8 and 10 are expressed in the anterior part of the second heart field that constitutes a population of cardiac progenitor cells contributing to the arterial pole of the heart. Previous studies of hypomorphic and conditional Fgf8 mutants show disrupted outflow tract (OFT) and right ventricle (RV) development, whereas Fgf10 mutants do not have detectable OFT defects.
Objectives: Our aim was to investigate functional overlap between Fgf8 and Fgf10 during formation of the arterial pole.
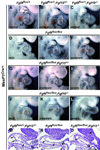
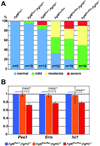
Methods and results: We generated mesodermal Fgf8; Fgf10 compound mutants with MesP1Cre. The OFT/RV morphology in these mutants was affected with variable penetrance; however, the incidence of embryos with severely affected OFT/RV morphology was significantly increased in response to decreasing Fgf8 and Fgf10 gene dosage. Fgf8 expression in the pharyngeal arch ectoderm is important for development of the pharyngeal arch arteries and their derivatives. We now show that Fgf8 deletion in the mesoderm alone leads to pharyngeal arch artery phenotypes and that these vascular phenotypes are exacerbated by loss of Fgf10 function in the mesodermal core of the arches.
Conclusions: These results show functional overlap of FGF8 and FGF10 signaling from second heart field mesoderm during development of the OFT/RV, and from pharyngeal arch mesoderm during pharyngeal arch artery formation, highlighting the sensitivity of these key aspects of cardiovascular development to FGF dosage.
Figures


Similar articles
-
An Fgf8 mouse mutant phenocopies human 22q11 deletion syndrome.Development. 2002 Oct;129(19):4591-603. doi: 10.1242/dev.129.19.4591. Development. 2002. PMID: 12223415 Free PMC article.
-
Fgf8 is required for pharyngeal arch and cardiovascular development in the mouse.Development. 2002 Oct;129(19):4613-25. doi: 10.1242/dev.129.19.4613. Development. 2002. PMID: 12223417
-
A genetic link between Tbx1 and fibroblast growth factor signaling.Development. 2002 Oct;129(19):4605-11. doi: 10.1242/dev.129.19.4605. Development. 2002. PMID: 12223416
-
Hoxa3 and signaling molecules involved in aortic arch patterning and remodeling.Cell Tissue Res. 2009 May;336(2):165-78. doi: 10.1007/s00441-009-0760-7. Epub 2009 Mar 17. Cell Tissue Res. 2009. PMID: 19290546 Review.
-
New developments in the second heart field.Differentiation. 2012 Jul;84(1):17-24. doi: 10.1016/j.diff.2012.03.003. Epub 2012 Apr 21. Differentiation. 2012. PMID: 22521611 Review.
Cited by
-
ISL1 directly regulates FGF10 transcription during human cardiac outflow formation.PLoS One. 2012;7(1):e30677. doi: 10.1371/journal.pone.0030677. Epub 2012 Jan 27. PLoS One. 2012. PMID: 22303449 Free PMC article.
-
Hey2 enhancer activity defines unipotent progenitors for left ventricular cardiomyocytes in juxta-cardiac field of early mouse embryo.Proc Natl Acad Sci U S A. 2023 Sep 12;120(37):e2307658120. doi: 10.1073/pnas.2307658120. Epub 2023 Sep 5. Proc Natl Acad Sci U S A. 2023. PMID: 37669370 Free PMC article.
-
Mutations in Hnrnpa1 cause congenital heart defects.JCI Insight. 2018 Jan 25;3(2):e98555. doi: 10.1172/jci.insight.98555. eCollection 2018 Jan 25. JCI Insight. 2018. PMID: 29367466 Free PMC article.
-
Fibroblast growth factor-8 inhibits oxidative stress-induced apoptosis in H9c2 cells.Mol Cell Biochem. 2017 Jan;425(1-2):77-84. doi: 10.1007/s11010-016-2863-2. Epub 2016 Nov 1. Mol Cell Biochem. 2017. PMID: 27804049
-
Sonic hedgehog protein regulates fibroblast growth factor 8 expression in metanephric explant culture from BALB/c mice: Possible mechanisms associated with renal morphogenesis.Mol Med Rep. 2016 Oct;14(4):2929-36. doi: 10.3892/mmr.2016.5614. Epub 2016 Aug 9. Mol Med Rep. 2016. PMID: 27510750 Free PMC article.
References
-
- Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–e181. - PubMed
-
- Nakajima Y, Yamagishi T, Hokari S, Nakamura H. Mechanisms involved in valvuloseptal endocardial cushion formation in early cardiogenesis: roles of transforming growth factor (TGF)-beta and bone morphogenetic protein (BMP) Anat Rec. 2000;258:119–127. - PubMed
-
- Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet. 2005;6:826–835. - PubMed
-
- Franco D, Meilhac SM, Christoffels VM, Kispert A, Buckingham M, Kelly RG. Left and right ventricular contributions to the formation of the interventricular septum in the mouse heart. Dev Biol. 2006;294:366–375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

