Declines in mortality rates and changes in causes of death in HIV-1-infected children during the HAART era
- PMID: 20035164
- PMCID: PMC2801894
- DOI: 10.1097/QAI.0b013e3181b9869f
Declines in mortality rates and changes in causes of death in HIV-1-infected children during the HAART era
Abstract
Context: Introduction of highly active antiretroviral therapy has significantly decreased mortality in HIV-1-infected adults and children. Although an increase in non-HIV-related mortality has been noted in adults, data in children are limited.
Objectives: To evaluate changes in causes and risk factors for death among HIV-1-infected children in Pediatric AIDS Clinical Trials Group 219/219C.
Design, setting, and participants: Multicenter, prospective cohort study designed to evaluate long-term outcomes in HIV-1-exposed and infected US children. There were 3553 HIV-1-infected children enrolled and followed up between April 1993 and December 2006, with primary cause of mortality identified in the 298 observed deaths.
Main outcome measures: Mortality rates per 100 child-years overall and by demographic factors; survival estimates by birth cohort; and hazard ratios for mortality by various demographic, health, and antiretroviral treatment factors were determined.
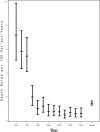
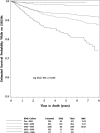
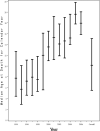
Results: Among 3553 HIV-1-infected children followed up for a median of 5.3 years, 298 deaths occurred. Death rates significantly decreased between 1994 and 2000, from 7.2 to 0.8 per 100 person-years, and remained relatively stable through 2006. After adjustment for other covariates, increased risk of death was identified for those with low CD4 and AIDS-defining illness at entry. Decreased risks of mortality were identified for later birth cohorts, and for time-dependent initiation of highly active antiretroviral therapy (hazard ratio 0.54, P < 0.001). The most common causes of death were "End-stage AIDS" (N = 48, 16%) and pneumonia (N = 41, 14%). The proportion of deaths due to opportunistic infections (OIs) declined from 37% in 1994-1996 to 24% after 2000. All OI mortality declined during the study period. However, a greater decline was noted for deaths due to Mycobacterium avium complex and cryptosporidium. Deaths from "End-stage AIDS," sepsis and renal failure increased.
Conclusions: Overall death rates declined from 1993 to 2000 but have since stabilized at rates about 30 times higher than for the general US pediatric population. Deaths due to OIs have declined, but non-AIDS-defining infections and multiorgan failure remain major causes of mortality in HIV-1-infected children.
Figures
References
-
- Gortmaker SL, Hughes M, Cervic J, Brady M, Johnson GM, Seage GR, 3rd, Song LY, Dankner WM, Oleske JM. Pediatric AIDS Clinical Trials Group Protocol 219 Team. Effect of combination therapy including protease inhibitors on mortality among children and adolescents infected with HIV-1. N Engl J Med. 2001;345:1522–1528. - PubMed
-
- Patel K, Hernán M, Williams P, Seeger J, McIntosh K, Van Dyke R. Seage III GR for the PACTG219/219C Study Team. Long-term Effectiveness of Highly Active Antiretroviral Therapy (HAART) on the Survival of Children and Adolescents infected with HIV-1. Clinical Infectious Diseases. 2008;46:507–515. - PubMed
-
- Abrams EF, Weedon J, Bertolli J, Bornschlegel K, Cervia J, Mendez H, Lambert G, Singh T, Thomas P. New York City Pediatric Surveillance of Disease Consortium, Centers for Disease Control and Prevention. Aging cohort of perinatally human immunodeficiency virus-infected children in New York City. New York City Pediatric Surveillance of Disease Consortium. Pediatr Infect Dis J. 2001;20:511–517. - PubMed
-
- deMartino M, Tovo PA, Balducci M, Galli L, Gabiano C, Rezza G, Pezzoti P. Reduction in mortality with availability of antiretroviral therapy for children with perinatal HIV-1 infection. Italian Register for HIV Infection in Children and the Italian National AIDS Registry. JAMA. 2000;284:190–197. - PubMed
-
- Judd A, Doerholt K, Tookey PA, et al. Moribidy, mortality and response to treatment in children in the United Kingdom and Ireland with perinatally-acquired HIV infection during 1996–2006: planning for teenage and adult care. Clin Infect Dis. 2007;45:918–924. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

