Respiration in adipocytes is inhibited by reactive oxygen species
- PMID: 20035277
- PMCID: PMC6154476
- DOI: 10.1038/oby.2009.456
Respiration in adipocytes is inhibited by reactive oxygen species
Abstract
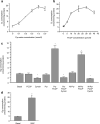
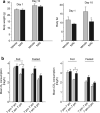
It is a desirable goal to stimulate fuel oxidation in adipocytes and shift the balance toward less fuel storage and more burning. To understand this regulatory process, respiration was measured in primary rat adipocytes, mitochondria, and fat-fed mice. Maximum O(2) consumption, in vitro, was determined with a chemical uncoupler of oxidative phosphorylation (carbonylcyanide p-trifluoromethoxyphenylhydrazone (FCCP)). The adenosine triphosphate/adenosine diphosphate (ATP/ADP) ratio was measured by luminescence. Mitochondria were localized by confocal microscopy with MitoTracker Green and their membrane potential (Delta psi(M)) measured using tetramethylrhodamine ethyl ester perchlorate (TMRE). The effect of N-acetylcysteine (NAC) on respiration and body composition in vivo was assessed in mice. Addition of FCCP collapsed Delta psi(M) and decreased the ATP/ADP ratio. However, we demonstrated the same rate of adipocyte O(2) consumption in the absence or presence of fuels and FCCP. Respiration was only stimulated when reactive oxygen species (ROS) were scavenged by pyruvate or NAC: other fuels or fuel combinations had little effect. Importantly, the ROS scavenging role of pyruvate was not affected by rotenone, an inhibitor of mitochondrial complex I. In addition, mice that consumed NAC exhibited increased O(2) consumption and decreased body fat in vivo. These studies suggest for the first time that adipocyte O(2) consumption may be inhibited by ROS, because pyruvate and NAC stimulated respiration. ROS inhibition of O(2) consumption may explain the difficulty to identify effective strategies to increase fat burning in adipocytes. Stimulating fuel oxidation in adipocytes by decreasing ROS may provide a novel means to shift the balance from fuel storage to fuel burning.
Conflict of interest statement
The authors declared no conflict of interest.
Figures


Similar articles
-
Redox regulation of endogenous substrate oxidation by cardiac mitochondria.Am J Physiol Heart Circ Physiol. 2006 Sep;291(3):H1436-45. doi: 10.1152/ajpheart.01292.2005. Epub 2006 Apr 14. Am J Physiol Heart Circ Physiol. 2006. PMID: 16617125
-
Regulation of pyruvate oxidation in blowfly flight muscle mitochondria: requirement for ADP.Arch Biochem Biophys. 1984 Nov 1;234(2):382-93. doi: 10.1016/0003-9861(84)90284-4. Arch Biochem Biophys. 1984. PMID: 6497378
-
Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species.J Neurosci. 2004 Sep 8;24(36):7779-88. doi: 10.1523/JNEUROSCI.1899-04.2004. J Neurosci. 2004. PMID: 15356189 Free PMC article.
-
New control of mitochondrial membrane potential and ROS formation--a hypothesis.Biol Chem. 2001 Dec;382(12):1629-36. doi: 10.1515/BC.2001.198. Biol Chem. 2001. PMID: 11843176 Review.
-
The mystery of reactive oxygen species derived from cell respiration.Acta Biochim Pol. 2004;51(1):223-9. Acta Biochim Pol. 2004. PMID: 15094844 Review.
Cited by
-
Protective Effects of Curcumin in Cardiovascular Diseases-Impact on Oxidative Stress and Mitochondria.Cells. 2022 Jan 20;11(3):342. doi: 10.3390/cells11030342. Cells. 2022. PMID: 35159155 Free PMC article. Review.
-
10E,12Z-conjugated linoleic acid impairs adipocyte triglyceride storage by enhancing fatty acid oxidation, lipolysis, and mitochondrial reactive oxygen species.J Lipid Res. 2013 Nov;54(11):2964-78. doi: 10.1194/jlr.M035188. Epub 2013 Aug 17. J Lipid Res. 2013. PMID: 23956445 Free PMC article.
-
N-Acetylcysteine affects obesity-related protein expression in 3T3-L1 adipocytes.Redox Rep. 2013;18(6):210-8. doi: 10.1179/1351000213Y.0000000066. Redox Rep. 2013. PMID: 24112955 Free PMC article.
-
Metabolic and Epigenetic Regulation by Estrogen in Adipocytes.Front Endocrinol (Lausanne). 2022 Feb 22;13:828780. doi: 10.3389/fendo.2022.828780. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35273571 Free PMC article. Review.
-
Withania somnifera Extract Enhances Energy Expenditure via Improving Mitochondrial Function in Adipose Tissue and Skeletal Muscle.Nutrients. 2020 Feb 7;12(2):431. doi: 10.3390/nu12020431. Nutrients. 2020. PMID: 32046183 Free PMC article.
References
-
- McGarry JD, Takabayashi Y, Foster DW. The role of malonyl-coa in the coordination of fatty acid synthesis and oxidation in isolated rat hepatocytes. J Biol Chem. 1978;253:8294–8300. - PubMed
-
- Wang YL, Guo W, Zang Y, et al. Acyl coenzyme a synthetase regulation: putative role in long-chain acyl coenzyme a partitioning. Obes Res. 2004;12:1781–1788. - PubMed
-
- Wang T, Zang Y, Ling W, Corkey BE, Guo W. Metabolic partitioning of endogenous fatty acid in adipocytes. Obes Res. 2003;11:880–887. - PubMed
-
- Zang Y, Wang T, Xie W, et al. Regulation of acetyl CoA carboxylase and carnitine palmitoyl transferase-1 in rat adipocytes. Obes Res. 2005;13:1530–1539. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

