The role of synapsins in neuronal development
- PMID: 20035364
- PMCID: PMC11115787
- DOI: 10.1007/s00018-009-0227-8
The role of synapsins in neuronal development
Abstract
The synapsins, the first identified synaptic vesicle-specific proteins, are phosphorylated on multiple sites by a number of protein kinases and are involved in neurite outgrowth and synapse formation as well as in synaptic transmission. In mammals, the synapsin family consists of at least 10 isoforms encoded by 3 distinct genes and composed by a mosaic of conserved and variable domains. The synapsins are highly conserved evolutionarily, and orthologues have been found in invertebrates and lower vertebrates. Within nerve terminals, synapsins are implicated in multiple interactions with presynaptic proteins and the actin cytoskeleton. Via these interactions, synapsins control several mechanisms important for neuronal homeostasis. In this review, we describe the main functional features of the synapsins, in relation to the complex role played by these phosphoproteins in neuronal development.
Figures


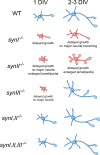
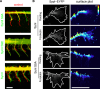
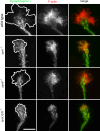
Similar articles
-
The synapsins: key actors of synapse function and plasticity.Prog Neurobiol. 2010 Aug;91(4):313-48. doi: 10.1016/j.pneurobio.2010.04.006. Epub 2010 May 10. Prog Neurobiol. 2010. PMID: 20438797 Review.
-
Synapsin knockdown is associated with decreased neurite outgrowth, functional synaptogenesis impairment, and fast high-frequency neurotransmitter release.J Neurosci Res. 2015 Oct;93(10):1492-506. doi: 10.1002/jnr.23624. Epub 2015 Jul 27. J Neurosci Res. 2015. PMID: 26213348
-
Synapsin Isoforms Regulating GABA Release from Hippocampal Interneurons.J Neurosci. 2016 Jun 22;36(25):6742-57. doi: 10.1523/JNEUROSCI.0011-16.2016. J Neurosci. 2016. PMID: 27335405 Free PMC article.
-
Molecular determinants of synapsin targeting to presynaptic terminals.J Neurosci. 2004 Apr 7;24(14):3711-20. doi: 10.1523/JNEUROSCI.5225-03.2004. J Neurosci. 2004. PMID: 15071120 Free PMC article.
-
Synapsins as regulators of neurotransmitter release.Philos Trans R Soc Lond B Biol Sci. 1999 Feb 28;354(1381):269-79. doi: 10.1098/rstb.1999.0378. Philos Trans R Soc Lond B Biol Sci. 1999. PMID: 10212475 Free PMC article. Review.
Cited by
-
Identification of β-Dystrobrevin as a Direct Target of miR-143: Involvement in Early Stages of Neural Differentiation.PLoS One. 2016 May 25;11(5):e0156325. doi: 10.1371/journal.pone.0156325. eCollection 2016. PLoS One. 2016. PMID: 27223470 Free PMC article.
-
A modular chemoenzymatic cascade strategy for the structure-customized assembly of ganglioside analogs.Commun Chem. 2024 Jan 18;7(1):17. doi: 10.1038/s42004-024-01102-9. Commun Chem. 2024. PMID: 38238524 Free PMC article.
-
Late recruitment of synapsin to nascent synapses is regulated by Cdk5.Cell Rep. 2013 Apr 25;3(4):1199-212. doi: 10.1016/j.celrep.2013.03.031. Epub 2013 Apr 18. Cell Rep. 2013. PMID: 23602570 Free PMC article.
-
The presynaptic machinery at the synapse of C. elegans.Invert Neurosci. 2018 Mar 12;18(2):4. doi: 10.1007/s10158-018-0207-5. Invert Neurosci. 2018. PMID: 29532181 Free PMC article. Review.
-
Involvement of synaptic genes in the pathogenesis of autism spectrum disorders: the case of synapsins.Front Pediatr. 2014 Sep 4;2:94. doi: 10.3389/fped.2014.00094. eCollection 2014. Front Pediatr. 2014. PMID: 25237665 Free PMC article. Review.
References
-
- De Camilli P, Cameron R, Greengard P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. I. Its general distribution in synapses of the central and peripheral nervous system demonstrated by immunofluorescence in frozen and plastic sections. J Cell Biol. 1983;96:1337–1354. doi: 10.1083/jcb.96.5.1337. - DOI - PMC - PubMed
-
- De Camilli P, Harris SM, Jr, Huttner WB, Greengard P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. II. Its specific association with synaptic vesicles demonstrated by immunocytochemistry in agarose-embedded synaptosomes. J Cell Biol. 1983;96:1355–1373. doi: 10.1083/jcb.96.5.1355. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

