Platelet aggregation inhibitors from hematophagous animals
- PMID: 20035779
- PMCID: PMC2888830
- DOI: 10.1016/j.toxicon.2009.12.003
Platelet aggregation inhibitors from hematophagous animals
Abstract
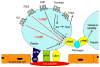
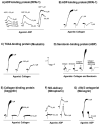
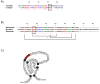
Salivary glands from blood-sucking animals (e.g., mosquitoes, bugs, sand flies, fleas, ticks, leeches, hookworms, bats) are a rich source of bioactive molecules that counteract hemostasis in a redundant and synergistic manner. This review discusses recent progress in the identification of salivary inhibitors of platelet aggregation, their molecular characterization, and detailed mechanism of action. Diversity of inhibitors is remarkable, with distinct families of proteins characterized as apyrases that enzymatically degrade ADP or as collagen-binding proteins that prevent its interaction with vWF, or platelet integrin α2β1 or GPVI. Molecules that bind ADP, TXA(2), epinephrine, or serotonin with high affinity have also been cloned, expressed, and their structure determined. In addition, a repertoire of antithrombins and an increasingly number of RGD and non-RGD disintegrins targeting platelet αIIbβ3 have been reported. Moreover, metalloproteases with fibrinogen(olytic) activity and PAF phosphorylcholine hydrolase are enzymes that have been recruited to the salivary gland to block platelet aggregation. Platelet inhibitory prostaglandins, lysophosphatydilcholine, adenosine, and nitric oxide (NO)-carrying proteins are other notable examples of molecules from hematophagous salivary secretions (herein named sialogenins) with antihemostatic properties. Sialogenins have been employed as tools in biochemistry and cell biology and also display potential therapeutic applications.
Published by Elsevier Ltd.
Conflict of interest statement
Figures

Similar articles
-
Disintegrins from hematophagous sources.Toxins (Basel). 2012 May;4(5):296-322. doi: 10.3390/toxins4050296. Epub 2012 Apr 26. Toxins (Basel). 2012. PMID: 22778902 Free PMC article. Review.
-
Anti-thrombosis repertoire of blood-feeding horsefly salivary glands.Mol Cell Proteomics. 2009 Sep;8(9):2071-9. doi: 10.1074/mcp.M900186-MCP200. Epub 2009 Jun 16. Mol Cell Proteomics. 2009. PMID: 19531497 Free PMC article.
-
A novel family of RGD-containing disintegrins (Tablysin-15) from the salivary gland of the horsefly Tabanus yao targets αIIbβ3 or αVβ3 and inhibits platelet aggregation and angiogenesis.Thromb Haemost. 2011 Jun;105(6):1032-45. doi: 10.1160/TH11-01-0029. Epub 2011 Apr 7. Thromb Haemost. 2011. PMID: 21475772 Free PMC article.
-
Functional characterization of a salivary apyrase from the sand fly, Phlebotomus duboscqi, a vector of Leishmania major.J Insect Physiol. 2009 Nov;55(11):1044-9. doi: 10.1016/j.jinsphys.2009.07.010. Epub 2009 Aug 11. J Insect Physiol. 2009. PMID: 19651132 Free PMC article.
-
Exogenous Integrin αIIbβ3 Inhibitors Revisited: Past, Present and Future Applications.Int J Mol Sci. 2021 Mar 25;22(7):3366. doi: 10.3390/ijms22073366. Int J Mol Sci. 2021. PMID: 33806083 Free PMC article. Review.
Cited by
-
Profiling of human acquired immunity against the salivary proteins of Phlebotomus papatasi reveals clusters of differential immunoreactivity.Am J Trop Med Hyg. 2014 May;90(5):923-938. doi: 10.4269/ajtmh.13-0130. Epub 2014 Mar 10. Am J Trop Med Hyg. 2014. PMID: 24615125 Free PMC article.
-
Molecular identification of Phlebotomus kandelakii apyrase and assessment of the immunogenicity of its recombinant protein in BALB/c mice.Sci Rep. 2023 May 30;13(1):8766. doi: 10.1038/s41598-023-36037-z. Sci Rep. 2023. PMID: 37253833 Free PMC article.
-
The "Vampirome": Transcriptome and proteome analysis of the principal and accessory submaxillary glands of the vampire bat Desmodus rotundus, a vector of human rabies.J Proteomics. 2013 Apr 26;82:288-319. doi: 10.1016/j.jprot.2013.01.009. Epub 2013 Feb 11. J Proteomics. 2013. PMID: 23411029 Free PMC article.
-
Induced Transient Immune Tolerance in Ticks and Vertebrate Host: A Keystone of Tick-Borne Diseases?Front Immunol. 2021 Feb 12;12:625993. doi: 10.3389/fimmu.2021.625993. eCollection 2021. Front Immunol. 2021. PMID: 33643313 Free PMC article. Review.
-
Evidence for a lectin specific for sulfated glycans in the salivary gland of the malaria vector, Anopheles gambiae.PLoS One. 2014 Sep 10;9(9):e107295. doi: 10.1371/journal.pone.0107295. eCollection 2014. PLoS One. 2014. PMID: 25207644 Free PMC article.
References
-
- Aljamali M, Bowman AS, Dillwith JW, Tucker JS, Yates GW, Essenberg RC, Sauer JR. Identity and synthesis of prostaglandins in the lone star tick, Amblyomma americanum (L.), as assessed by radio-immunoassay and gas chromatography/mass spectrometry. Insect Biochem Mol Biol. 2002;32:331–341. - PubMed
-
- Andersen JF, Francischetti IM, Valenzuela JG, Schuck P, Ribeiro JM. Inhibition of hemostasis by a high affinity biogenic amine-binding protein from the saliva of a blood-feeding insect. J Biol Chem. 2003;278:4611–4617. - PubMed
-
- Barnes CS, Krafft B, Frech M, Hofmann UR, Papendieck A, Dahlems U, Gellissen G, Hoylaerts MF. Production and characterization of saratin, an inhibitor of von Willebrand factor-dependent platelet adhesion to collagen. Semin Thromb Hemost. 2001;27:337–348. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

