Cognitive recovery in the aged rat after stroke and anti-Nogo-A immunotherapy
- PMID: 20035795
- PMCID: PMC2831114
- DOI: 10.1016/j.bbr.2009.12.015
Cognitive recovery in the aged rat after stroke and anti-Nogo-A immunotherapy
Abstract
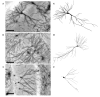
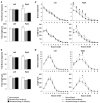
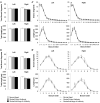
We have previously shown that immunotherapy directed against the protein Nogo-A leads to recovery on a skilled forelimb reaching task in rats after sensorimotor cortex stroke, which correlated with axonal and dendritic plasticity. Here we investigated anti-Nogo-A immunotherapy as an intervention to improve performance on a spatial memory task in aged rats after stroke, and whether cognitive recovery was correlated with structural plasticity. Aged rats underwent a unilateral distal permanent middle cerebral artery occlusion and one week later were treated with an anti-Nogo-A or control antibody. Nine weeks post-stroke, treated rats and normal aged rats were tested on the Morris water maze task. Following testing rats were sacrificed and brains processed for the Golgi-Cox method. Hippocampal CA3 and CA1 pyramidal and dentate gyrus granule cells were examined for dendritic length and number of branch segments, and CA3 and CA1 pyramidal cells were examined for spine density and morphology. Anti-Nogo-A immunotherapy given one week following stroke in aged rats improved performance on the reference memory portion of the Morris water maze task. However, this improved performance was not correlated with structural changes in the hippocampal neurons examined. Our finding of improved performance on the Morris water maze in aged rats after stroke and treatment with anti-Nogo-A immunotherapy demonstrates the promising therapeutic potential for anti-Nogo-A immunotherapy to treat cognitive deficits after stroke. The identification of sites of axonal and dendritic plasticity in the aged brain after stroke and treatment with anti-Nogo-A immunotherapy is still under investigation.
Copyright 2010 Elsevier B.V. All rights reserved.
Figures




References
-
- Recommendations for Standards Regarding Preclinical Neuroprotective and Restorative Drug Development. Stroke. 1999;30:2752–2758. - PubMed
-
- Andersen MB, Zimmer J, Sams-Dodd F. Specific behavioral effects related to age and cerebral ischemia in rats. Pharmacol Biochem Behav. 1999;62:673–682. - PubMed
-
- Badan I, Buchhold B, Hamm A, Gratz M, Walker LC, Platt D, Kessler C, Popa-Wagner A. Accelerated glial reactivity to stroke in aged rats correlates with reduced functional recovery. J Cereb Blood Flow Metab. 2003;23:845–854. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

