A detailed characterisation of the distribution and presentation of DNA vaccine encoded antigen
- PMID: 20035828
- PMCID: PMC2824851
- DOI: 10.1016/j.vaccine.2009.11.014
A detailed characterisation of the distribution and presentation of DNA vaccine encoded antigen
Abstract
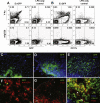
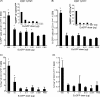
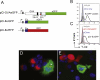
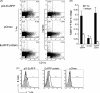
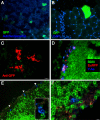
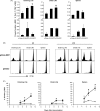
The association between plasmid DNA distribution, the amount of Ag produced, Ag persistence and the identity and localisation of cells presenting DNA-encoded Ag all have important consequences for both quantitative and qualitative aspects of T cell responses induced by DNA vaccines. Using a variety of approaches to detect and quantify the uptake of injected DNA, and the production and presentation of DNA-encoded antigen, we report that injected DNA vaccines rapidly enter the peripheral blood from the injection site and also reach muscle-draining lymph nodes directly as free DNA. 24h after plasmid injection, MHCII(+)CD11b(+)B220(-)CD11c(low/-) cells in the draining and distal LNs and spleen contain pDNA. Interestingly, we also observed pDNA(+)MHCII(low/-)CD11b(+) within the bone marrow. Concomitantly, we detected Ag-containing/expressing cells at both the injection site and in draining lymph nodes. Three days after plasmid injection we detected rare pMHC(+)CD11c(+) cells within secondary lymphoid tissue and simultaneously observed Ag-specific CD4(+) T cell accumulation and blastogenesis in these tissues. Our results show that the events that determine the induction of DNA vaccine immune responses occur within days of DNA injection and that the response becomes systemic very rapidly, possibly with involvement from resident BM cells.
Copyright (c) 2009 Elsevier Ltd. All rights reserved.
Figures


References
-
- Itano A.A., McSorley S.J., Reinhardt R.L., Ehst B.D., Ingulli E., Rudensky A.Y. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19(1):47–57. - PubMed
-
- Rudensky A., Rath S., Preston-Hurlburt P., Murphy D.B., Janeway C.A., Jr. On the complexity of self. Nature. 1991;353(6345):660–662. - PubMed
-
- Hou S., Hyland L., Ryan K.W., Portner A., Doherty P.C. Virus-specific CD8+ T-cell memory determined by clonal burst size. Nature. 1994;369(6482):652–654. - PubMed
-
- Cho T.H., Chang S.H., Jan Y.S. Dose-dependent selective priming of Th1 and Th2 immune responses is achieved only by an antigen with an affinity over a certain threshold level. Mol Cells. 2000;10(6):695–704. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

