Remote sites of structural atrophy predict later amyloid formation in a mouse model of Alzheimer's disease
- PMID: 20035883
- PMCID: PMC2823970
- DOI: 10.1016/j.neuroimage.2009.12.070
Remote sites of structural atrophy predict later amyloid formation in a mouse model of Alzheimer's disease
Abstract
Magnetic resonance (MR) imaging can provide a longitudinal view of neurological disease through repeated imaging of patients at successive stages of impairment. Until recently, the difficulty of manual delineation has limited volumetric analyses of MR data sets to a few select regions and a small number of subjects. Increased throughput offered by faster imaging methods, automated segmentation, and deformation-based morphometry have recently been applied to overcome this limitation with mouse models of neurological conditions. We use automated analyses to produce an unbiased view of volumetric changes in a transgenic mouse model for Alzheimer's disease (AD) at two points in the progression of disease: immediately before and shortly after the onset of amyloid formation. In addition to the cortex and hippocampus, where atrophy has been well documented in AD patients, we identify volumetric losses in the pons and substantia nigra where neurodegeneration has not been carefully examined. We find that deficits in cortical volume precede amyloid formation in this mouse model, similar to presymptomatic atrophy seen in patients with familial AD. Unexpectedly, volumetric losses identified by MR outside of the forebrain predict locations of future amyloid formation, such as the inferior colliculus and spinal nuclei, which develop pathology at very late stages of disease. Our work provides proof-of-principle that MR microscopy can expand our view of AD by offering a complete and unbiased examination of volumetric changes that guide us in revisiting the canonical neuropathology.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures



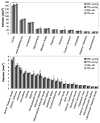

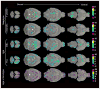



References
-
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. - PMC - PubMed
-
- Busatto GF, Diniz BS, Zanetti MV. Voxel-based morphometry in Alzheimer’s disease. Expert Rev Neurother. 2008;8:1691–1702. - PubMed
-
- Busch C, Bohl J, Ohm TG. Spatial, temporal and numeric analysis of Alzheimer changes in the nucleus coeruleus. Neurobiol Aging. 1997;18:401–406. - PubMed
-
- Chung MK, Worsley KJ, Paus T, Cherif C, Collins DL, Giedd JN, Rapoport JL, Evans AC. A unified statistical approach to deformation-based morphometry. Neuroimage. 2001;14:595–606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

