Safety, pharmacokinetics, and preliminary activity of the anti-IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing's sarcoma: a phase 1 expansion cohort study
- PMID: 20036194
- PMCID: PMC2941877
- DOI: 10.1016/S1470-2045(09)70354-7
Safety, pharmacokinetics, and preliminary activity of the anti-IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing's sarcoma: a phase 1 expansion cohort study
Abstract
Background: Figitumumab is a fully human IgG2 monoclonal antibody targeting the insulin-like growth-factor-1 receptor (IGF-1R). Preclinical data suggest a dependence on insulin-like growth-factor signalling for sarcoma subtypes, including Ewing's sarcoma, and early reports show antitumour activity of IGF-1R-targeting drugs in these diseases.
Methods: Between January, 2006, and August, 2008, patients with refractory, advanced sarcomas received figitumumab (20 mg/kg) in two single-stage expansion cohorts within a solid-tumour phase 1 trial. The first cohort (n=15) included patients with multiple sarcoma subtypes, age 18 years or older, and the second cohort (n=14) consisted of patients with refractory Ewing's sarcoma, age 9 years or older. The primary endpoint was to assess the safety and tolerability of figitumumab. Secondary endpoints included pharmacokinetic profiling and preliminary antitumour activity (best response by Response Evaluation Criteria in Solid Tumours [RECIST]) in evaluable patients who received at least one dose of medication. This study is registered with ClinicalTrials.gov, number NCT00474760.
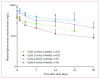
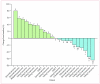
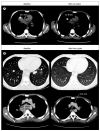
Findings: 29 patients, 16 of whom had Ewing's sarcoma, were enrolled and received a total of 177 cycles of treatment (median 2, mean 6.1, range 1-24). Grade 3 deep venous thrombosis, grade 3 back pain, and grade 3 vomiting were each noted once in individual patients; one patient had grade 3 increases in aspartate aminotransferase and gammaglutamyltransferase concentrations. This patient also had grade 4 increases in alanine aminotransferase concentrations. The only other grade 4 adverse event was raised concentrations of uric acid, noted in one patient. Pharmacokinetics were comparable between patients with sarcoma and those with other solid tumours. 28 patients were assessed for response; two patients, both with Ewing's sarcoma, had objective responses (one complete response and one partial response) and eight patients had disease stabilisation (six with Ewing's sarcoma, one with synovial sarcoma, and one with fibrosarcoma) lasting 4 months or longer.
Interpretation: Figitumumab is well tolerated and has antitumour activity in Ewing's sarcoma, warranting further investigation in this disease.
Funding: Pfizer Global Research and Development.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures
Comment in
-
IGF-1R targeted treatment of sarcoma.Lancet Oncol. 2010 Feb;11(2):105-6. doi: 10.1016/S1470-2045(09)70391-2. Epub 2009 Dec 23. Lancet Oncol. 2010. PMID: 20036195 No abstract available.
References
-
- Hartmann JT. Systemic treatment options for patients with refractory adult-type sarcoma beyond anthracyclines. Anticancer Drugs. 2007;18:245–54. - PubMed
-
- Helman LJ, Meltzer P. Mechanisms of sarcoma development. Nat Rev Cancer. 2003;3:685–94. - PubMed
-
- Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–28. - PubMed
-
- Samani AA, Yakar S, LeRoith D, et al. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28:20–47. - PubMed
-
- Massague J, Czech MP. The subunit structures of two distinct receptors for insulin-like growth factors I and II and their relationship to the insulin receptor. J Biol Chem. 1982;257:5038–45. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

