The effects of sympathetic outflow on upregulation of vanilloid receptors TRPV(1) in primary afferent neurons evoked by intradermal capsaicin
- PMID: 20036240
- PMCID: PMC2824038
- DOI: 10.1016/j.expneurol.2009.12.011
The effects of sympathetic outflow on upregulation of vanilloid receptors TRPV(1) in primary afferent neurons evoked by intradermal capsaicin
Abstract
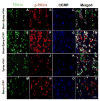
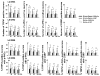
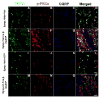
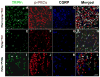
The vanilloid receptor TRPV(1) is a key nociceptive molecule located in primary afferent nociceptive neurons in dorsal root ganglia (DRG) for initiating neurogenic inflammation and pain. Our recent study demonstrates that up-regulation of TRPV(1) receptors by intradermal injection of capsaicin is modulated by activation of the protein kinase C (PKC) cascade. Neurogenic inflammation and pain resulting from capsaicin injection are sympathetically dependent, responding to norepinephrine, adenosine 5'-triphosphate (ATP) and/or neuropeptide Y released from sympathetic efferents. In a rat model of acute neurogenic inflammatory pain produced by capsaicin injection, we used immunofluorescence and Western blots combined with pharmacology and surgical sympathectomies to analyze whether the capsaicin-evoked up-regulation of TRPV(1) in DRG neurons is affected by sympathetic outflow by way of activating the PKC cascade. Sympathetic denervation reduced significantly the capsaicin-evoked expressions of TRPV(1), calcitonin gene-related peptide and/or phosphorylated PKC and their co-expression. These reductions could be restored by exogenous pretreatment with an analog of ATP, alpha,beta-methylene ATP. Inhibition of PKC with chelerythrine chloride prevented the ATP effect. Consistent results were obtained from experiments in which capsaicin-evoked changes in cutaneous inflammation (vasodilation and edema) were examined after sympathetic denervation, and the effects of the above pharmacological manipulations were evaluated. Our findings suggest that the capsaicin-evoked up-regulation of TRPV(1) receptors in DRG neurons is modulated sympathetically by the action of ATP released from sympathetic efferents to activate the PKC cascade. Thus, this study proposes a potential new mechanism of sympathetic modulation of neurogenic inflammation.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures

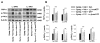

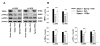

Similar articles
-
Increases in transient receptor potential vanilloid-1 mRNA and protein in primary afferent neurons stimulated by protein kinase C and their possible role in neurogenic inflammation.J Neurosci Res. 2009 Feb;87(2):482-94. doi: 10.1002/jnr.21844. J Neurosci Res. 2009. PMID: 18752301 Free PMC article.
-
Sensitization of primary afferent nociceptors induced by intradermal capsaicin involves the peripheral release of calcitonin gene-related Peptide driven by dorsal root reflexes.J Pain. 2008 Dec;9(12):1155-68. doi: 10.1016/j.jpain.2008.06.011. Epub 2008 Aug 13. J Pain. 2008. PMID: 18701354 Free PMC article.
-
Neurokinin 2 receptor-mediated activation of protein kinase C modulates capsaicin responses in DRG neurons from adult rats.Eur J Neurosci. 2008 Jun;27(12):3171-81. doi: 10.1111/j.1460-9568.2008.06267.x. Eur J Neurosci. 2008. PMID: 18598261 Free PMC article.
-
Roles of TRPV1 and neuropeptidergic receptors in dorsal root reflex-mediated neurogenic inflammation induced by intradermal injection of capsaicin.Mol Pain. 2007 Oct 25;3:30. doi: 10.1186/1744-8069-3-30. Mol Pain. 2007. PMID: 17961222 Free PMC article.
-
Potentiation of glutamatergic synaptic transmission by protein kinase C-mediated sensitization of TRPV1 at the first sensory synapse.J Physiol. 2007 Jun 1;581(Pt 2):631-47. doi: 10.1113/jphysiol.2006.118620. Epub 2007 Mar 15. J Physiol. 2007. PMID: 17363391 Free PMC article.
Cited by
-
Epac-protein kinase C alpha signaling in purinergic P2X3R-mediated hyperalgesia after inflammation.Pain. 2016 Jul;157(7):1541-1550. doi: 10.1097/j.pain.0000000000000547. Pain. 2016. PMID: 26963850 Free PMC article.
-
Noradrenaline regulates substance P release from rat dorsal root ganglion neurons in vitro.Neurosci Bull. 2011 Oct;27(5):300-6. doi: 10.1007/s12264-011-1034-4. Neurosci Bull. 2011. PMID: 21934725 Free PMC article.
-
Anti-superoxide and anti-peroxynitrite strategies in pain suppression.Biochim Biophys Acta. 2012 May;1822(5):815-21. doi: 10.1016/j.bbadis.2011.12.008. Epub 2011 Dec 19. Biochim Biophys Acta. 2012. PMID: 22200449 Free PMC article.
-
Involvement of subtypes γ and ε of protein kinase C in colon pain induced by formalin injection.Neurosignals. 2011;19(3):142-50. doi: 10.1159/000328311. Epub 2011 Jun 23. Neurosignals. 2011. PMID: 21701146 Free PMC article.
-
Minocycline inhibits the enhancement of antidromic primary afferent stimulation-evoked vasodilation following intradermal capsaicin injection.Neurosci Lett. 2010 Sep 27;482(2):177-81. doi: 10.1016/j.neulet.2010.07.031. Epub 2010 Jul 21. Neurosci Lett. 2010. PMID: 20654697 Free PMC article.
References
-
- Aoki Y, Ohtori S, Takahashi K, Ino H, Douya H, Ozawa T, Saito T, Moriya H. Expression and Co-expression of VR1, CGRP, and IB-4-binding glycoprotein in dorsal root ganglion neurons in rats: differences between the disc afferents and the cutaneous afferents. Spine. 2005;30:1496–1500. - PubMed
-
- Burnstock G. Purine-mediated signaling in pain and visceral perception. Trends Pharmacol Sci. 2001;22:182–188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

