Molecular and structural insight into proNGF engagement of p75NTR and sortilin
- PMID: 20036257
- PMCID: PMC2847487
- DOI: 10.1016/j.jmb.2009.12.030
Molecular and structural insight into proNGF engagement of p75NTR and sortilin
Abstract
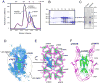
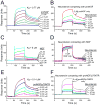
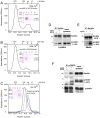
Nerve growth factor (NGF) is initially synthesized as a precursor, proNGF, that is cleaved to release its C-terminal mature form. Recent studies suggested that proNGF is not an inactive precursor but acts as a signaling ligand distinct from its mature counterpart. proNGF and mature NGF initiate opposing biological responses by utilizing both distinct and shared receptor components. In this study, we carried out structural and biochemical characterization of proNGF interactions with p75NTR and sortilin. We crystallized proNGF complexed to p75NTR and present the structure at 3.75-A resolution. The structure reveals a 2:2 symmetric binding mode, as compared with the asymmetric structure of a previously reported crystal structure of mature NGF complexed to p75NTR and the 2:2 symmetric complex of neurotrophin-3 (NT-3) and p75NTR. Here, we discuss the possible origins and implications of the different stoichiometries. In the proNGF-p75NTR complex, the pro regions of proNGF are mostly disordered and two hairpin loops (loop 2) at the top of the NGF dimer have undergone conformational changes in comparison with mature NT structures, suggesting possible interactions with the propeptide. We further explored the binding characteristics of proNGF to sortilin using surface plasmon resonance and cell-based assays and determined that calcium ions promote the formation of a stable ternary complex of proNGF-sortilin-p75NTR. These results, together with those of previous structural and mechanistic studies of NT-receptor interactions, suggest the potential for distinct signaling activities through p75NTR mediated by different NT-induced conformational changes.
(c) 2009 Elsevier Ltd. All rights reserved.
Figures




Similar articles
-
Intrinsic structural disorder of mouse proNGF.Proteins. 2009 Jun;75(4):990-1009. doi: 10.1002/prot.22311. Proteins. 2009. PMID: 19089979
-
[NRH2 induces cell apoptosis of cerebral tissues around hematomas after intracerebral hemorrhage through up-regulating proNGF, sortilin and p75NTR expressions].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2015 Apr;31(4):532-6, 539. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2015. PMID: 25854576 Chinese.
-
Sortilin is essential for proNGF-induced neuronal cell death.Nature. 2004 Feb 26;427(6977):843-8. doi: 10.1038/nature02319. Nature. 2004. PMID: 14985763
-
NGF and ProNGF: Regulation of neuronal and neoplastic responses through receptor signaling.Adv Biol Regul. 2015 May;58:16-27. doi: 10.1016/j.jbior.2014.11.003. Epub 2014 Nov 20. Adv Biol Regul. 2015. PMID: 25491371 Free PMC article. Review.
-
The proNGF-p75NTR-sortilin signalling complex as new target for the therapeutic treatment of Parkinson's disease.CNS Neurol Disord Drug Targets. 2008 Dec;7(6):512-23. doi: 10.2174/187152708787122923. CNS Neurol Disord Drug Targets. 2008. PMID: 19128208 Review.
Cited by
-
Pro-nerve growth factor induces autocrine stimulation of breast cancer cell invasion through tropomyosin-related kinase A (TrkA) and sortilin protein.J Biol Chem. 2012 Jan 13;287(3):1923-31. doi: 10.1074/jbc.M110.211714. Epub 2011 Nov 29. J Biol Chem. 2012. PMID: 22128158 Free PMC article.
-
Precursor of brain-derived neurotrophic factor (proBDNF) forms a complex with Huntingtin-associated protein-1 (HAP1) and sortilin that modulates proBDNF trafficking, degradation, and processing.J Biol Chem. 2011 May 6;286(18):16272-84. doi: 10.1074/jbc.M110.195347. Epub 2011 Feb 28. J Biol Chem. 2011. PMID: 21357693 Free PMC article.
-
Trk Receptors and Neurotrophin Cross-Interactions: New Perspectives Toward Manipulating Therapeutic Side-Effects.Front Mol Neurosci. 2017 May 3;10:130. doi: 10.3389/fnmol.2017.00130. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28515680 Free PMC article.
-
Death Domain Signaling by Disulfide-Linked Dimers of the p75 Neurotrophin Receptor Mediates Neuronal Death in the CNS.J Neurosci. 2016 May 18;36(20):5587-95. doi: 10.1523/JNEUROSCI.4536-15.2016. J Neurosci. 2016. PMID: 27194337 Free PMC article.
-
proBDNF inhibits the proliferation and migration of OLN‑93 oligodendrocytes.Mol Med Rep. 2018 Oct;18(4):3809-3817. doi: 10.3892/mmr.2018.9407. Epub 2018 Aug 21. Mol Med Rep. 2018. PMID: 30132570 Free PMC article.
References
-
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–62. - PubMed
-
- Snider WD. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994;77:627–38. - PubMed
-
- Edwards RH, Selby MJ, Garcia PD, Rutter WJ. Processing of the native nerve growth factor precursor to form biologically active nerve growth factor. J Biol Chem. 1988;263:6810–5. - PubMed
-
- Rattenholl A, Lilie H, Grossmann A, Stern A, Schwarz E, Rudolph R. The pro-sequence facilitates folding of human nerve growth factor from Escherichia coli inclusion bodies. Eur J Biochem. 2001;268:3296–303. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

