Aldo-keto reductase 1C3 expression in MCF-7 cells reveals roles in steroid hormone and prostaglandin metabolism that may explain its over-expression in breast cancer
- PMID: 20036328
- PMCID: PMC2819162
- DOI: 10.1016/j.jsbmb.2009.12.009
Aldo-keto reductase 1C3 expression in MCF-7 cells reveals roles in steroid hormone and prostaglandin metabolism that may explain its over-expression in breast cancer
Abstract
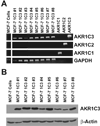
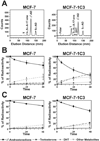
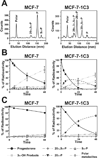
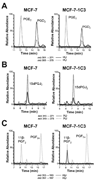
Aldo-keto reductase (AKR) 1C3 (type 5 17beta-hydroxysteroid dehydrogenase and prostaglandin F synthase), may stimulate proliferation via steroid hormone and prostaglandin (PG) metabolism in the breast. Purified recombinant AKR1C3 reduces PGD(2) to 9alpha,11beta-PGF(2), Delta(4)-androstenedione to testosterone, progesterone to 20alpha-hydroxyprogesterone, and to a lesser extent, estrone to 17beta-estradiol. We established MCF-7 cells that stably express AKR1C3 (MCF-7-AKR1C3 cells) to model its over-expression in breast cancer. AKR1C3 expression increased steroid conversion by MCF-7 cells, leading to a pro-estrogenic state. Unexpectedly, estrone was reduced fastest by MCF-7-AKR1C3 cells when compared to other substrates at 0.1muM. MCF-7-AKR1C3 cells proliferated three times faster than parental cells in response to estrone and 17beta-estradiol. AKR1C3 therefore represents a potential target for attenuating estrogen receptor alpha induced proliferation. MCF-7-AKR1C3 cells also reduced PGD(2), limiting its dehydration to form PGJ(2) products. The AKR1C3 product was confirmed as 9alpha,11beta-PGF(2) and quantified with a stereospecific stable isotope dilution liquid chromatography-mass spectrometry method. This method will allow the examination of the role of AKR1C3 in endogenous prostaglandin formation in response to inflammatory stimuli. Expression of AKR1C3 reduced the anti-proliferative effects of PGD(2) on MCF-7 cells, suggesting that AKR1C3 limits peroxisome proliferator activated receptor gamma (PPARgamma) signaling by reducing formation of 15-deoxy-Delta(12,14)-PGJ(2) (15dPGJ(2)).
Keywords: 17β-Hydroxysteroid dehydrogenase; estrogen receptor; peroxisome proliferator activated receptor γ; prostaglandin D2; prostaglandin F synthase.
Figures


Similar articles
-
Type 5 17beta-hydroxysteroid dehydrogenase/prostaglandin F synthase (AKR1C3): role in breast cancer and inhibition by non-steroidal anti-inflammatory drug analogs.Chem Biol Interact. 2009 Mar 16;178(1-3):221-7. doi: 10.1016/j.cbi.2008.10.024. Epub 2008 Nov 1. Chem Biol Interact. 2009. PMID: 19010312 Free PMC article.
-
Aldo-keto reductase 1C3 is overexpressed in skin squamous cell carcinoma (SCC) and affects SCC growth via prostaglandin metabolism.Exp Dermatol. 2014 Aug;23(8):573-8. doi: 10.1111/exd.12468. Epub 2014 Jul 16. Exp Dermatol. 2014. PMID: 24917395 Free PMC article.
-
Structure-function aspects and inhibitor design of type 5 17beta-hydroxysteroid dehydrogenase (AKR1C3).Mol Cell Endocrinol. 2001 Jan 22;171(1-2):137-49. doi: 10.1016/s0303-7207(00)00426-3. Mol Cell Endocrinol. 2001. PMID: 11165022
-
Inhibitors of type 5 17β-hydroxysteroid dehydrogenase (AKR1C3): overview and structural insights.J Steroid Biochem Mol Biol. 2011 May;125(1-2):95-104. doi: 10.1016/j.jsbmb.2010.11.004. Epub 2010 Nov 16. J Steroid Biochem Mol Biol. 2011. PMID: 21087665 Free PMC article. Review.
-
Steroid hormone transforming aldo-keto reductases and cancer.Ann N Y Acad Sci. 2009 Feb;1155:33-42. doi: 10.1111/j.1749-6632.2009.03700.x. Ann N Y Acad Sci. 2009. PMID: 19250190 Free PMC article. Review.
Cited by
-
Structural and Functional Biology of Aldo-Keto Reductase Steroid-Transforming Enzymes.Endocr Rev. 2019 Apr 1;40(2):447-475. doi: 10.1210/er.2018-00089. Endocr Rev. 2019. PMID: 30137266 Free PMC article. Review.
-
Modulated expression of genes encoding estrogen metabolizing enzymes by G1-phase cyclin-dependent kinases 6 and 4 in human breast cancer cells.PLoS One. 2014 May 21;9(5):e97448. doi: 10.1371/journal.pone.0097448. eCollection 2014. PLoS One. 2014. PMID: 24848372 Free PMC article.
-
The aldo-keto reductases (AKRs): Overview.Chem Biol Interact. 2015 Jun 5;234:236-46. doi: 10.1016/j.cbi.2014.09.024. Epub 2014 Oct 7. Chem Biol Interact. 2015. PMID: 25304492 Free PMC article. Review.
-
Identification of genes associated with castration‑resistant prostate cancer by gene expression profile analysis.Mol Med Rep. 2017 Nov;16(5):6803-6813. doi: 10.3892/mmr.2017.7488. Epub 2017 Sep 13. Mol Med Rep. 2017. PMID: 28901445 Free PMC article.
-
Genetic association study of Preterm birth and Gestational age in a population-based case-control study in Peru: Genetics of PTB and GA in pregnant women in Peru.medRxiv [Preprint]. 2023 Nov 22:2023.11.22.23298891. doi: 10.1101/2023.11.22.23298891. medRxiv. 2023. Update in: J Neonatal Perinatal Med. 2024;17(5):689-704. doi: 10.3233/NPM-230228. PMID: 38045296 Free PMC article. Updated. Preprint.
References
-
- Labrie F, Luu-The V, Lin SX, Simard J, Labrie C, El-Alfy M, Pelletier G, Belanger A. Intracrinology: role of the family of 17β-hydroxysteroid dehydrogenases in human physiology and disease. J Mol Endocrinol. 2000;25(1):1–16. - PubMed
-
- Suzuki T, Miki Y, Nakamura Y, Moriya T, Ito K, Ohuchi N, Sasano H. Sex steroid-producing enzymes in human breast cancer. Endocr Relat Cancer. 2005;12(4):701–720. - PubMed
-
- Penning TM, Burczynski ME, Jez JM, Hung CF, Lin HK, Ma H, Moore M, Palackal N, Ratnam K. Human 3α-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the aldo-keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem J. 2000;351(Pt 1):67–77. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

