Cyclophosphamide induces bone marrow to yield higher numbers of precursor dendritic cells in vitro capable of functional antigen presentation to T cells in vivo
- PMID: 20036354
- PMCID: PMC2821961
- DOI: 10.1016/j.cellimm.2009.11.011
Cyclophosphamide induces bone marrow to yield higher numbers of precursor dendritic cells in vitro capable of functional antigen presentation to T cells in vivo
Abstract
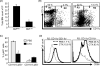
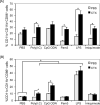
We have shown recently that cyclophosphamide (CTX) treatment induced a marked increase in the numbers of immature dendritic cells (DCs) in blood, coinciding with enhanced antigen-specific responses of the adoptively transferred CD8(+) T cells. Because this DC expansion was preceded by DC proliferation in bone marrow (BM), we tested whether BM post CTX treatment can generate higher numbers of functional DCs. BM was harvested three days after treatment of C57BL/6 mice with PBS or CTX and cultured with GM-CSF/IL-4 in vitro. Compared with control, BM from CTX-treated mice showed faster generation and yielded higher numbers of DCs with superior activation in response to toll-like receptor (TLR) agonists. Vaccination with peptide-pulsed DCs generated from BM from CTX-treated mice induced comparable adjuvant effects to those induced by control DCs. Taken together, post CTX BM harbors higher numbers of DC precursors capable of differentiating into functional DCs, which be targeted to create host microenvironment riches in activated DCs upon treatment with TLR agonists.
Published by Elsevier Inc.
Figures




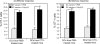
References
-
- Jurado JM, Sanchez A, Pajares B, Perez E, Alonso L, Alba E. Combined oral cyclophosphamide and bevacizumab in heavily pre-treated ovarian cancer. Clin Transl Oncol. 2008;10:583–6. - PubMed
-
- Rosell R, Moreno I, Maestre J, Olazabal A, Carles J, Barnadas A, Abad-Esteve A, Ribelles N, Canela M. Cyclophosphamide and ifosfamide combination as neoadjuvant chemotherapy for locally advanced nonsmall-cell lung cancer: a meta-analytic review. J Surg Oncol. 1990;45:124–30. - PubMed
-
- Saxton ML, Longo DL, Wetzel HE, Tribble H, Alvord WG, Kwak LW, Leonard AS, Ullmann CD, Curti BD, Ochoa AC. Adoptive transfer of anti-CD3-activated CD4+ T cells plus cyclophosphamide and liposome-encapsulated interleukin-2 cure murine MC-38 and 3LL tumors and establish tumor-specific immunity. Blood. 1997;89:2529–36. - PubMed
-
- Curti BD, Ochoa AC, Powers GC, Kopp WC, Alvord WG, Janik JE, Gause BL, Dunn B, Kopreski MS, Fenton R, Zea A, Dansky-Ullmann C, Strobl S, Harvey L, Nelson E, Sznol M, Longo DL. Phase I trial of anti-CD3-stimulated CD4+ T cells, infusional interleukin-2, and cyclophosphamide in patients with advanced cancer. J Clin Oncol. 1998;16:2752–60. - PubMed
-
- Bracci L, Moschella F, Sestili P, La Sorsa V, Valentini M, Canini I, Baccarini S, Maccari S, Ramoni C, Belardelli F, Proietti E. Cyclophosphamide enhances the antitumor efficacy of adoptively transferred immune cells through the induction of cytokine expression, B-cell and T-cell homeostatic proliferation, and specific tumor infiltration. Clin Cancer Res. 2007;13:644–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

