Epac, not PKA catalytic subunit, is required for 3T3-L1 preadipocyte differentiation
- PMID: 20036887
- PMCID: PMC2799683
- DOI: 10.2741/e99
Epac, not PKA catalytic subunit, is required for 3T3-L1 preadipocyte differentiation
Abstract
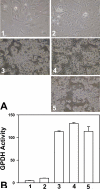
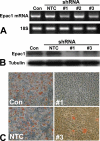
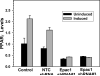
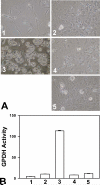
Cyclic AMP plays a critical role in adipocyte differentiation and maturation. However, it is not clear which of the two intracellular cAMP receptors, exchange protein directly activated by cAMP/cAMP-regulated guanine nucleotide exchange factor or protein kinase A/cAMP-dependent protein kinase, is essential for cAMP-mediated adipocyte differentiation. In this study, we utilized a well-defined adipose differentiation model system, the murine preadipocyte line 3T3-L1, to address this issue. We showed that knocking down Epac expression in 3T3-L1 cells using lentiviral based small hairpin RNAs down-regulated peroxisome proliferator-activated receptor gamma expression and dramatically inhibited adipogenic conversion of 3T3-L1 cells while inhibiting PKA catalytic subunit activity by two mechanistically distinct inhibitors, heat stable protein kinase inhibitor and H89, had no effect on 3T3-L1 adipocyte differentiation. Moreover, cAMP analog selectively activating Epac was not able to stimulate adipogenic conversion. Our study demonstrated that while PKA catalytic activity is dispensable, activation of Epac is necessary but not sufficient for adipogenic conversion of 3T3-L1 cells.
Figures
Similar articles
-
Exchange protein activated by cyclic AMP is involved in the regulation of adipogenic genes during 3T3-L1 fibroblasts differentiation.Dev Growth Differ. 2014 Feb;56(2):143-51. doi: 10.1111/dgd.12114. Epub 2014 Jan 20. Dev Growth Differ. 2014. PMID: 24444094
-
Cyclic AMP (cAMP)-mediated stimulation of adipocyte differentiation requires the synergistic action of Epac- and cAMP-dependent protein kinase-dependent processes.Mol Cell Biol. 2008 Jun;28(11):3804-16. doi: 10.1128/MCB.00709-07. Epub 2008 Apr 7. Mol Cell Biol. 2008. PMID: 18391018 Free PMC article.
-
Simvastatin suppresses leptin expression in 3T3-L1 adipocytes via activation of the cyclic AMP-PKA pathway induced by inhibition of protein prenylation.J Biochem. 2009 Jun;145(6):771-81. doi: 10.1093/jb/mvp035. Epub 2009 Mar 2. J Biochem. 2009. PMID: 19254925
-
PKA-dependent and independent cAMP signaling in 3T3-L1 fibroblasts differentiation.Mol Cell Endocrinol. 2009 Jan 27;298(1-2):42-7. doi: 10.1016/j.mce.2008.10.023. Epub 2008 Oct 25. Mol Cell Endocrinol. 2009. PMID: 19010385
-
Epac and PKA: a tale of two intracellular cAMP receptors.Acta Biochim Biophys Sin (Shanghai). 2008 Jul;40(7):651-62. doi: 10.1111/j.1745-7270.2008.00438.x. Acta Biochim Biophys Sin (Shanghai). 2008. PMID: 18604457 Free PMC article. Review.
Cited by
-
Activation of β-adrenoceptors by dobutamine may induce a higher expression of peroxisome proliferator-activated receptors δ (PPARδ) in neonatal rat cardiomyocytes.ScientificWorldJournal. 2012;2012:248320. doi: 10.1100/2012/248320. Epub 2012 May 15. ScientificWorldJournal. 2012. PMID: 22666095 Free PMC article.
-
Anti-hyperlipidemic Effect of Polyphenol Extract (Seapolynol(™)) and Dieckol Isolated from Ecklonia cava in in vivo and in vitro Models.Prev Nutr Food Sci. 2012 Mar;17(1):1-7. doi: 10.3746/pnf.2012.17.1.001. Prev Nutr Food Sci. 2012. PMID: 24471056 Free PMC article.
-
Protein-altering variants associated with body mass index implicate pathways that control energy intake and expenditure in obesity.Nat Genet. 2018 Jan;50(1):26-41. doi: 10.1038/s41588-017-0011-x. Epub 2017 Dec 22. Nat Genet. 2018. PMID: 29273807 Free PMC article.
-
Enhanced leptin sensitivity, reduced adiposity, and improved glucose homeostasis in mice lacking exchange protein directly activated by cyclic AMP isoform 1.Mol Cell Biol. 2013 Mar;33(5):918-26. doi: 10.1128/MCB.01227-12. Epub 2012 Dec 21. Mol Cell Biol. 2013. PMID: 23263987 Free PMC article.
-
Current methods of adipogenic differentiation of mesenchymal stem cells.Stem Cells Dev. 2011 Oct;20(10):1793-804. doi: 10.1089/scd.2011.0040. Epub 2011 Jun 20. Stem Cells Dev. 2011. PMID: 21526925 Free PMC article. Review.
References
-
- Cowherd RM, Lyle RE, McGehee RE., Jr Molecular regulation of adipocyte differentiation. Semin Cell Dev Biol. 1999;10:3–10. - PubMed
-
- Green H, Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974;3:127–133. - PubMed
-
- Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783–809. - PubMed
-
- Green H, Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975;5:19–27. - PubMed
-
- Cao Z, Umek RM, McKnight SL. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991;5:1538–1552. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

