Ligand trapping by cytochrome c oxidase: implications for gating at the catalytic center
- PMID: 20037139
- PMCID: PMC2836058
- DOI: 10.1074/jbc.M109.078618
Ligand trapping by cytochrome c oxidase: implications for gating at the catalytic center
Abstract
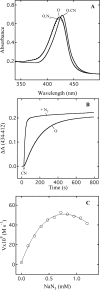
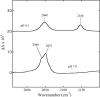
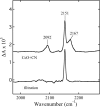
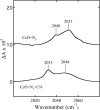
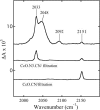
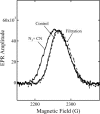
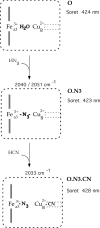
Cytochrome c oxidase is a member of the heme-copper family of oxygen reductases in which electron transfer is linked to the pumping of protons across the membrane. Neither the redox center(s) associated with proton pumping nor the pumping mechanism presumably common to all heme-copper oxidases has been established. A possible conformational coupling between the catalytic center (Fe(a3)(3+)-Cu(B)(2+)) and a protein site has been identified earlier from ligand binding studies, whereas a structural change initiated by azide binding to the protein has been proposed to facilitate the access of cyanide to the catalytic center of the oxidized bovine enzyme. Here we show that cytochrome oxidase pretreated with a low concentration of azide exhibits a significant increase in the apparent rate of cyanide binding relative to that of free enzyme. However, this increase in rate does not reflect a conformational change enhancing the rapid formation of a Fe(a3)(3+)-CN-Cu(B)(2+) complex. Instead the cyanide-induced transition of a preformed Fe(a3)(3+)-N(3)-Cu(B)(2+) to the ternary complex of Fe(a3)(3+)-N(3) Cu(B)(2+)-CN is the most likely reason for the observed acceleration. Significantly, the slow rate of azide release from the ternary complex indicates that cyanide ligated to Cu(B) blocks a channel between the catalytic site and the solvent. The results suggest that there is a pathway that originates at Cu(B) and that, during catalysis, ligands present at this copper center control access to the iron of heme a(3) from the bulk medium.
Figures
Similar articles
-
FTIR detection of protonation/deprotonation of key carboxyl side chains caused by redox change of the Cu(A)-heme a moiety and ligand dissociation from the heme a3-Cu(B) center of bovine heart cytochrome c oxidase.J Am Chem Soc. 2003 Jun 18;125(24):7209-18. doi: 10.1021/ja021302z. J Am Chem Soc. 2003. PMID: 12797794
-
EPR evidence of cyanide binding to the Mn(Mg) center of cytochrome c oxidase: support for Cu(A)-Mg involvement in proton pumping.Biochemistry. 2009 Jan 20;48(2):328-35. doi: 10.1021/bi801391r. Biochemistry. 2009. PMID: 19108635 Free PMC article.
-
X-ray structural analyses of azide-bound cytochrome c oxidases reveal that the H-pathway is critically important for the proton-pumping activity.J Biol Chem. 2018 Sep 21;293(38):14868-14879. doi: 10.1074/jbc.RA118.003123. Epub 2018 Aug 3. J Biol Chem. 2018. PMID: 30077971 Free PMC article.
-
Active site structure of the aa3 quinol oxidase of Acidianus ambivalens.Biochim Biophys Acta. 2004 Apr 12;1655(1-3):306-20. doi: 10.1016/j.bbabio.2003.08.011. Biochim Biophys Acta. 2004. PMID: 15100046 Review.
-
Cooperative coupling and role of heme a in the proton pump of heme-copper oxidases.Biochimie. 1998 Oct;80(10):821-36. doi: 10.1016/s0300-9084(00)88877-x. Biochimie. 1998. PMID: 9893941 Review.
Cited by
-
Rhodamine-based sensor for real-time imaging of mitochondrial ATP in living fibroblasts.Biochim Biophys Acta Bioenerg. 2017 Dec;1858(12):999-1006. doi: 10.1016/j.bbabio.2017.09.004. Epub 2017 Sep 22. Biochim Biophys Acta Bioenerg. 2017. PMID: 28947254 Free PMC article.
-
Redirecting Intermediary Metabolism to Counteract Cyanide Poisoning.FASEB J. 2025 Jun 30;39(12):e70709. doi: 10.1096/fj.202400230RR. FASEB J. 2025. PMID: 40497726 Free PMC article. Review.
-
Proton-coupled electron transfer.Chem Rev. 2007 Nov;107(11):5004-64. doi: 10.1021/cr0500030. Chem Rev. 2007. PMID: 17999556 Free PMC article. Review. No abstract available.
References
-
- Wikstrom M. K. (1977) Nature 266, 271–273 - PubMed
-
- Kadenbach B., Jarausch J., Hartmann R., Merle P. (1983) Anal. Biochem. 129, 517–521 - PubMed
-
- Tsukihara T., Aoyama H., Yamashita E., Tomizaki T., Yamaguchi H., Shinzawa-Itoh K., Nakashima R., Yaono R., Yoshikawa S. (1996) Science 272, 1136–1144 - PubMed
-
- Day E. P., Peterson J., Sendova M. S., Schoonover J., Palmer G. (1993) Biochemistry 32, 7855–7860 - PubMed
-
- Fann Y. C., Ahmed I., Blackburn N. J., Boswell J. S., Verkhovskaya M. L., Hoffman B. M., Wikström M. (1995) Biochemistry 34, 10245–10255 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

