Transforming growth factor-beta-activated kinase 1 is an essential regulator of myogenic differentiation
- PMID: 20037161
- PMCID: PMC2825435
- DOI: 10.1074/jbc.M109.064063
Transforming growth factor-beta-activated kinase 1 is an essential regulator of myogenic differentiation
Abstract
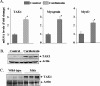
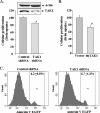
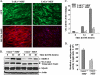
Satellite cells/myoblasts account for the majority of muscle regenerative potential in response to injury and muscular adaptation to exercise. Although the ability to influence this process would provide valuable benefits for treating a variety of patients suffering from muscle loss, the regulatory mechanisms of myogenesis are not completely understood. We have tested the hypothesis that transforming growth factor-beta-activated kinase 1 (TAK1) is an important regulator of skeletal muscle formation. TAK1 is expressed in proliferating C2C12 myoblasts, and its levels are reduced upon differentiation of myoblasts into myotubes. In vivo, TAK1 is predominantly expressed in developing skeletal muscle of young mice. However, the expression of TAK1 was significantly up-regulated in regenerating skeletal muscle of adult mice. Overexpression of a dominant negative mutant of TAK1 or knockdown of TAK1 inhibited the proliferation and differentiation of C2C12 myoblasts. TAK1 was required for the expression of myogenic regulatory factors in differentiating myoblasts. Genetic ablation of TAK1 also inhibited the MyoD-driven transformation of mouse embryonic fibroblasts into myotubes. Inhibition of TAK1 suppressed the differentiation-associated activation of p38 mitogen-activated protein kinase (MAPK) and Akt kinase. Overexpression of a constitutively active mutant of MAPK kinase 6 (MKK6, an upstream activator of p38 MAPK) but not constitutive active Akt restored the myogenic differentiation in TAK1-deficient mouse embryonic fibroblasts. Insulin growth factor 1-induced myogenic differentiation was also found to involve TAK1. Collectively, our results suggest that TAK1 is an important upstream regulator of skeletal muscle cell differentiation.
Figures





Similar articles
-
Emerging role of TAK1 in the regulation of skeletal muscle mass.Bioessays. 2023 Apr;45(4):e2300003. doi: 10.1002/bies.202300003. Epub 2023 Feb 15. Bioessays. 2023. PMID: 36789559 Free PMC article. Review.
-
TGF-β-activated kinase 1 (TAK1) and apoptosis signal-regulating kinase 1 (ASK1) interact with the promyogenic receptor Cdo to promote myogenic differentiation via activation of p38MAPK pathway.J Biol Chem. 2012 Apr 6;287(15):11602-15. doi: 10.1074/jbc.M112.351601. Epub 2012 Feb 15. J Biol Chem. 2012. PMID: 22337877 Free PMC article.
-
TRAF6 promotes myogenic differentiation via the TAK1/p38 mitogen-activated protein kinase and Akt pathways.PLoS One. 2012;7(4):e34081. doi: 10.1371/journal.pone.0034081. Epub 2012 Apr 4. PLoS One. 2012. PMID: 22496778 Free PMC article.
-
p-TAK1 acts as a switch between myoblast proliferation phase and differentiation phase in mdx mice via regulating HO-1 expression.Eur J Pharmacol. 2022 Oct 15;933:175277. doi: 10.1016/j.ejphar.2022.175277. Epub 2022 Sep 14. Eur J Pharmacol. 2022. PMID: 36113553
-
The p38 MAPK signaling pathway: a major regulator of skeletal muscle development.Mol Cell Endocrinol. 2006 Jun 27;252(1-2):224-30. doi: 10.1016/j.mce.2006.03.017. Epub 2006 Apr 27. Mol Cell Endocrinol. 2006. PMID: 16644098 Review.
Cited by
-
Emerging role of TAK1 in the regulation of skeletal muscle mass.Bioessays. 2023 Apr;45(4):e2300003. doi: 10.1002/bies.202300003. Epub 2023 Feb 15. Bioessays. 2023. PMID: 36789559 Free PMC article. Review.
-
APPL1 mediates adiponectin-stimulated p38 MAPK activation by scaffolding the TAK1-MKK3-p38 MAPK pathway.Am J Physiol Endocrinol Metab. 2011 Jan;300(1):E103-10. doi: 10.1152/ajpendo.00427.2010. Epub 2010 Oct 26. Am J Physiol Endocrinol Metab. 2011. PMID: 20978232 Free PMC article.
-
Mechanical loading and TGF-β change the expression of multiple miRNAs in tendon fibroblasts.J Appl Physiol (1985). 2012 Jul;113(1):56-62. doi: 10.1152/japplphysiol.00301.2012. Epub 2012 Apr 26. J Appl Physiol (1985). 2012. PMID: 22539168 Free PMC article.
-
TAK-1/p38/nNFκB signaling inhibits myoblast differentiation by increasing levels of Activin A.Skelet Muscle. 2012 Feb 7;2(1):3. doi: 10.1186/2044-5040-2-3. Skelet Muscle. 2012. PMID: 22313861 Free PMC article.
-
Regulation of cell-cell fusion by nanotopography.Sci Rep. 2016 Sep 12;6:33277. doi: 10.1038/srep33277. Sci Rep. 2016. PMID: 27615159 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

