Evaluation of the efficacy and cross-protectivity of recent human and swine vaccines against the pandemic (H1N1) 2009 virus infection
- PMID: 20037716
- PMCID: PMC2793524
- DOI: 10.1371/journal.pone.0008431
Evaluation of the efficacy and cross-protectivity of recent human and swine vaccines against the pandemic (H1N1) 2009 virus infection
Abstract
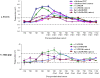
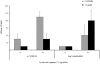
The current pandemic (H1N1) 2009 virus remains transmissible among humans worldwide with cases of reverse zoonosis, providing opportunities to produce more pathogenic variants which could pose greater human health concerns. To investigate whether recent seasonal human or swine H1N1 vaccines could induce cross-reactive immune responses against infection with the pandemic (H1N1) 2009 virus, mice, ferrets or mini-pigs were administered with various regimens (once or twice) and antigen content (1.77, 3.5 or 7.5 microg HA) of a-Brsibane/59/07, a-CAN01/04 or RgCA/04/09xPR8 vaccine. Receipt of a-CAN01/04 (2-doses) but not a-Brisbane/59/07 induced detectable but modest (20-40 units) cross-reactive serum antibody against CA/04/09 by hemagglutinin inhibition (HI) assays in mice. Only double administration (7.5 microg HA) of both vaccine in ferrets could elicit cross-reactivity (30-60 HI titers). Similar antigen content of a-CAN01/04 in mini-pigs also caused a modest approximately 30 HI titers (twice vaccinated). However, vaccine-induced antibody titers could not suppress active virus replication in the lungs (mice) or virus shedding (ferrets and pigs) of immunized hosts intranasally challenged with CA/04/09. Furthermore, neither ferrets nor swine could abrogate aerosol transmission of the virus into naïve contact animals. Altogether, these results suggest that neither recent human nor animal H1N1 vaccine could provide complete protectivity in all animal models. Thus, this study warrants the need for strain-specific vaccines that could yield the optimal protection desired for humans and/or animals.
Conflict of interest statement
Figures

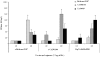

Similar articles
-
Recombinant soluble, multimeric HA and NA exhibit distinctive types of protection against pandemic swine-origin 2009 A(H1N1) influenza virus infection in ferrets.J Virol. 2010 Oct;84(19):10366-74. doi: 10.1128/JVI.01035-10. Epub 2010 Aug 4. J Virol. 2010. PMID: 20686020 Free PMC article.
-
Neuraminidase-Inhibiting Antibody Titers Correlate with Protection from Heterologous Influenza Virus Strains of the Same Neuraminidase Subtype.J Virol. 2018 Aug 16;92(17):e01006-18. doi: 10.1128/JVI.01006-18. Print 2018 Sep 1. J Virol. 2018. PMID: 29925654 Free PMC article.
-
Efficacy of seasonal live attenuated influenza vaccine against virus replication and transmission of a pandemic 2009 H1N1 virus in ferrets.Vaccine. 2011 Apr 5;29(16):2887-94. doi: 10.1016/j.vaccine.2011.02.014. Epub 2011 Feb 21. Vaccine. 2011. PMID: 21338676
-
Harnessing Local Immunity for an Effective Universal Swine Influenza Vaccine.Viruses. 2017 May 5;9(5):98. doi: 10.3390/v9050098. Viruses. 2017. PMID: 28475122 Free PMC article. Review.
-
Emergence and pandemic potential of swine-origin H1N1 influenza virus.Nature. 2009 Jun 18;459(7249):931-9. doi: 10.1038/nature08157. Nature. 2009. PMID: 19525932 Free PMC article. Review.
Cited by
-
Immunogenic and protective properties of the first Kazakhstan vaccine against pandemic influenza A (H1N1) pdm09 in ferrets.Virol Sin. 2012 Dec;27(6):345-52. doi: 10.1007/s12250-012-3272-7. Epub 2012 Nov 9. Virol Sin. 2012. PMID: 23180289 Free PMC article.
-
Immunogenicity of a monovalent 2009 influenza A (H1N1) vaccine in an immunocompromised population: a prospective study comparing HIV-infected adults with HIV-uninfected adults.Clin Infect Dis. 2011 Jan 1;52(1):138-46. doi: 10.1093/cid/ciq019. Epub 2010 Dec 7. Clin Infect Dis. 2011. PMID: 21148532 Free PMC article. Clinical Trial.
-
Two years after pandemic influenza A/2009/H1N1: what have we learned?Clin Microbiol Rev. 2012 Apr;25(2):223-63. doi: 10.1128/CMR.05012-11. Clin Microbiol Rev. 2012. PMID: 22491771 Free PMC article. Review.
-
An influenza A/H1N1/2009 hemagglutinin vaccine produced in Escherichia coli.PLoS One. 2010 Jul 22;5(7):e11694. doi: 10.1371/journal.pone.0011694. PLoS One. 2010. PMID: 20661476 Free PMC article.
-
Minipigs as an animal model for dermal vaccine delivery.Comp Med. 2014 Feb;64(1):50-4. Comp Med. 2014. PMID: 24512961 Free PMC article.
References
-
- Cohen J, Enserink M. Swine flu. After delays, WHO agrees: the 2009 pandemic has begun. Science. 2009;324:1496–1497. - PubMed
-
- Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–2615. - PubMed
-
- Shinde V, Bridges CB, Uyeki TM, Shu B, Balish A, et al. Triple-reassortant swine influenza A (H1) in humans in the United States, 2005-2009. N Engl J Med. 2009;360:2616–2625. - PubMed
-
- World Health Organization. New influenza A (H1N1) virus: global epidemiological situation, June 2009. Wkly Epidemiol Rec. 2009;84:249–257. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

