High harvest yield, high expansion, and phenotype stability of CD146 mesenchymal stromal cells from whole primitive human umbilical cord tissue
- PMID: 20037738
- PMCID: PMC2796378
- DOI: 10.1155/2009/789526
High harvest yield, high expansion, and phenotype stability of CD146 mesenchymal stromal cells from whole primitive human umbilical cord tissue
Abstract
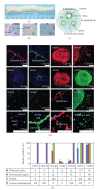
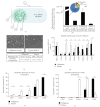
Human umbilical cord blood is an excellent primitive source of noncontroversial stem cells for treatment of hematologic disorders; meanwhile, new stem cell candidates in the umbilical cord (UC) tissue could provide therapeutic cells for nonhematologic disorders. We show novel in situ characterization to identify and localize a panel of some markers expressed by mesenchymal stromal cells (MSCs; CD44, CD105, CD73, CD90) and CD146 in the UC. We describe enzymatic isolation and purification methods of different UC cell populations that do not require manual separation of the vessels and stroma of the coiled, helical-like UC tissue. Unique quantitation of in situ cell frequency and stromal cell counts upon harvest illustrate the potential to obtain high numerical yields with these methods. UC stromal cells can differentiate to the osteogenic and chondrogenic lineages and, under specific culturing conditions, they exhibit high expandability with unique long-term stability of their phenotype. The remarkable stability of the phenotype represents a novel finding for human MSCs, from any source, and supports the use of these cells as highly accessible stromal cells for both basic studies and potentially therapeutic applications such as allogeneic clinical use for musculoskeletal disorders.
Figures


Similar articles
-
Equine Cord Blood Mesenchymal Stromal Cells Have Greater Differentiation and Similar Immunosuppressive Potential to Cord Tissue Mesenchymal Stromal Cells.Stem Cells Dev. 2019 Feb 1;28(3):227-237. doi: 10.1089/scd.2018.0135. Epub 2019 Jan 14. Stem Cells Dev. 2019. PMID: 30484372
-
Phenotypic Characterization of Adherent Cells Population CD34+ CD90+ CD105+ Derived from Wharton's Jelly.Med Sci Monit. 2017 Apr 19;23:1886-1895. doi: 10.12659/msm.902186. Med Sci Monit. 2017. PMID: 28422936 Free PMC article.
-
MSCs can be differentially isolated from maternal, middle and fetal segments of the human umbilical cord.Cytotherapy. 2016 Dec;18(12):1493-1502. doi: 10.1016/j.jcyt.2016.08.003. Epub 2016 Oct 7. Cytotherapy. 2016. PMID: 27727016
-
Expression profiles of human somatic mesenchymal stem cells derived from fresh endometrium, ectopic-endometrium and umbilical cord.Ginekol Pol. 2023;94(12):950-958. doi: 10.5603/gpl.93052. Epub 2023 Nov 7. Ginekol Pol. 2023. PMID: 37934895
-
Mesenchymal stem cell subpopulations: phenotype, property and therapeutic potential.Cell Mol Life Sci. 2016 Sep;73(17):3311-21. doi: 10.1007/s00018-016-2229-7. Epub 2016 May 3. Cell Mol Life Sci. 2016. PMID: 27141940 Free PMC article. Review.
Cited by
-
Mesenchymal stromal cell characteristics vary depending on their origin.Stem Cells Dev. 2013 Oct 1;22(19):2606-18. doi: 10.1089/scd.2013.0016. Epub 2013 Jun 22. Stem Cells Dev. 2013. PMID: 23676112 Free PMC article.
-
Isolation and characterization of human trophoblast side-population (SP) cells in primary villous cytotrophoblasts and HTR-8/SVneo cell line.PLoS One. 2011;6(7):e21990. doi: 10.1371/journal.pone.0021990. Epub 2011 Jul 7. PLoS One. 2011. PMID: 21760941 Free PMC article.
-
Umbilical cord-derived Wharton's jelly for regenerative medicine applications in orthopedic surgery: a systematic review protocol.J Orthop Surg Res. 2020 Nov 11;15(1):527. doi: 10.1186/s13018-020-02067-w. J Orthop Surg Res. 2020. PMID: 33176838 Free PMC article.
-
Umbilical cord as a mesenchymal stem cell source for treating joint pathologies.World J Orthop. 2011 Jun 18;2(6):43-50. doi: 10.5312/wjo.v2.i6.43. World J Orthop. 2011. PMID: 22474635 Free PMC article.
-
Umbilical Cord Stem Cell Lysate: A New Biologic Injectate for the Putative Treatment of Acute Temporomandibular Joint Inflammation.J Inflamm Res. 2023 Sep 27;16:4287-4300. doi: 10.2147/JIR.S420741. eCollection 2023. J Inflamm Res. 2023. PMID: 37791119 Free PMC article.
References
-
- Gluckman E, Rocha V. History of the clinical use of umbilical cord blood hematopoietic cells. Cytotherapy. 2005;7(3):219–227. - PubMed
-
- Kelly PF, Radtke S, von Kalle C, et al. Stem cell collection and gene transfer in fanconi anemia. Molecular Therapy. 2007;15(1):211–219. - PubMed
-
- Wagner JE, Rosenthal J, Sweetman R, et al. Successful transplantation of HLA-matched and HLA-mismatched umbilical cord blood from unrelated donors: analysis of engraftment and acute graft- versus-host disease. Blood. 1996;88(3):795–802. - PubMed
-
- Nauta AJ, Kruisselbrink AB, Lurvink E, et al. Enhanced engraftment of umbilical cord blood-derived stem cells in NOD/SCID mice by cotransplantation of a second unrelated cord blood unit. Experimental Hematology. 2005;33(10):1249–1256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
