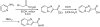Fully automated continuous flow synthesis of highly functionalized imidazo[1,2-a] heterocycles
- PMID: 20038130
- PMCID: PMC2814065
- DOI: 10.1021/ol902433a
Fully automated continuous flow synthesis of highly functionalized imidazo[1,2-a] heterocycles
Abstract
The first continuous flow synthesis of imidazo[1,2-a]pyridine-2-carboxylic acids directly from 2-aminopyridines and bromopyruvic acid has been developed, representing a significant advance over the corresponding in-flask method. The process was applied to the multistep synthesis of imidazo[1,2-a]pyridine-2-carboxamides, including a Mur ligase inhibitor, using a two microreactor, multistep continuous flow process without isolation of intermediates.
Figures
Similar articles
-
Direct arylation of imidazo[1,2-a]pyridine at C-3 with aryl iodides, bromides, and triflates via copper(I)-catalyzed C-H bond functionalization.Org Lett. 2012 Apr 6;14(7):1688-91. doi: 10.1021/ol300232a. Epub 2012 Mar 14. Org Lett. 2012. PMID: 22417233
-
One-step continuous flow synthesis of highly substituted pyrrole-3-carboxylic acid derivatives via in situ hydrolysis of tert-butyl esters.Org Lett. 2010 Nov 19;12(22):5182-5. doi: 10.1021/ol102216x. Epub 2010 Oct 21. Org Lett. 2010. PMID: 20964284 Free PMC article.
-
Synthesis of imidazopyridines from the Morita-Baylis-Hillman acetates of nitroalkenes and convenient access to Alpidem and Zolpidem.Org Lett. 2012 Sep 7;14(17):4580-3. doi: 10.1021/ol3020418. Epub 2012 Aug 24. Org Lett. 2012. PMID: 22920993
-
Synthesis of imidazo[1,2-a]pyridines: a decade update.Chem Commun (Camb). 2015 Jan 31;51(9):1555-75. doi: 10.1039/c4cc08495k. Chem Commun (Camb). 2015. PMID: 25407981 Review.
-
Pharmacological Potential and Synthetic Approaches of Imidazo[4,5-b]pyridine and Imidazo[4,5-c]pyridine Derivatives.Molecules. 2017 Mar 4;22(3):399. doi: 10.3390/molecules22030399. Molecules. 2017. PMID: 28273868 Free PMC article. Review.
Cited by
-
Harnessing Thin-Film Continuous-Flow Assembly Lines.Chemistry. 2016 Jul 25;22(31):10773-6. doi: 10.1002/chem.201602373. Epub 2016 Jun 22. Chemistry. 2016. PMID: 27198926 Free PMC article.
-
Continuous-flow synthesis of highly functionalized imidazo-oxadiazoles facilitated by microfluidic extraction.Beilstein J Org Chem. 2017 Feb 7;13:239-246. doi: 10.3762/bjoc.13.26. eCollection 2017. Beilstein J Org Chem. 2017. PMID: 28326132 Free PMC article.
-
A multi pathway coupled domino strategy: I2/TBHP-promoted synthesis of imidazopyridines and thiazoles via sp3, sp2 and sp C-H functionalization.RSC Adv. 2022 Feb 17;12(10):5919-5927. doi: 10.1039/d1ra07438e. eCollection 2022 Feb 16. RSC Adv. 2022. PMID: 35424560 Free PMC article.
-
A Combination of Flow and Batch Mode Processes for the Efficient Preparation of mGlu2/3 Receptor Negative Allosteric Modulators (NAMs).Tetrahedron. 2018 Jun 21;74(25):3165-3170. doi: 10.1016/j.tet.2018.03.068. Epub 2018 Mar 30. Tetrahedron. 2018. PMID: 30705468 Free PMC article.
-
One-pot synthesis of 2-phenylimidazo[1,2-α]pyridines from acetophenone, [Bmim]Br(3) and 2-aminopyridine under solvent-free conditions.Molecules. 2012 Nov 9;17(11):13368-75. doi: 10.3390/molecules171113368. Molecules. 2012. PMID: 23143149 Free PMC article.
References
-
- Schreiber SL. Science. 2000;287:1964. - PubMed
- Nicolaou KC, Zhong YL, Baran PS. Angew Chem Int Ed. 2000;39:622. - PubMed
- Nicolaou KC, Zhong YL, Baran PS. Angew Chem Int Ed. 2000;39:625. - PubMed
- Sheehan SM, Lalic G, Chen JS, Shair MD. Angew Chem Int Ed. 2000;39:2714. - PubMed
- Huryn DM, Cosford NDP. Annu Rep Med Chem. 2007;42:401. - PMC - PubMed
- Dobson CM. Nature. 2004;432:824. - PubMed
- Stockwell BR. Nature. 2004;432:846. - PMC - PubMed
- Lipinski C, Hopkins A. Nature. 2004;432:855. - PubMed
-
- Griffiths-Jones CM, Hopkin MD, Jönsson D, Ley SV, Tapolczay DJ, Vickerstaffe E, Ladlow M. J Comb Chem. 2007;9:422. - PubMed
- Baxendale IR, Ley SV, Smith CD, Tranmer GK. Chem Commun. 2006:4835. - PubMed
- Baumann M, Baxendale IR, Ley SV, Nikbin N, Smith CD, Tierney JP. Org Biomol Chem. 2008;6:1577. - PubMed
- Baumann M, Baxendale IR, Ley SV, Nikbin N, Smith CD. Org Biomol Chem. 2008;6:1587. - PubMed
- Brandt JC, Wirth T. Beilstein J Org Chem. 2009;5(No 30) - PMC - PubMed
- Hooper J, Watts P, Wiles C. Microfluidics Nanofluidics. 2008;5:595.
- Baumann M, Baxendale IR, Ley SV, Smith CD, Tranmer GK. Org Lett. 2006;8:5231. - PubMed
- Jas G, Kirschning A. Chem Eur J. 2003;9:5708. - PubMed
- Kirschning A, Solodenko W, Mennecke K. Chem Eur J. 2006;12:5972. - PubMed
-
- Mason BP, Price KE, Steinbacher JL, Bogdan AR, McQuade DT. Chem Rev. 2007;107:2300. - PubMed
- Baxendale IR, Hayward JJ, Lanners S, Ley SV, Smith CD. In: Microreactors in Organic Synthesis and Catalysis. Wirth T, editor. Wiley-VCH; Weinheim: 2008.
- Ehrfeld W, Hessel V, Lowe H. Microreactors: New Technology for Modern Chemistry. Wiley; Weinheim: 2000.
- Jähnisch K, Hessel V, Löwe H, Baerns M. Angew Chem Int Ed. 2004;43:406. - PubMed
- Csajági C, Borcsek B, Niesz K, Kovács I, Székelyhidi Z, Bajkó Z, Ürge L, Darvas F. Org Lett. 2008;10:1589. - PubMed
- Yoshida J, Nagaki A, Yamada T. Chem Eur J. 2008;14:7450. - PubMed
- Fukuyama T, Kobayashi M, Rahman MT, Kamata N, Ryu I. Org Lett. 2008;10:533. - PubMed
- Kundig P. Science. 2006;314:430. - PubMed
- Carrel FR, Geyer K, Codee JD, Seeberger PH. Org Lett. 2007;9:2285. - PubMed
- Watts P, Wiles C. Chem Commun. 2007:443. - PubMed
- Watts P, Wiles C. Org Biomol Chem. 2007;5:727. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources