The proteoglycan metabolism of articular cartilage in joint-scale culture
- PMID: 20038199
- PMCID: PMC2952130
- DOI: 10.1089/ten.TEA.2009.0663
The proteoglycan metabolism of articular cartilage in joint-scale culture
Abstract
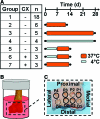
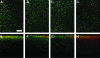
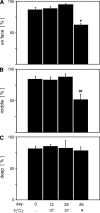
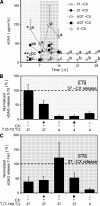
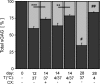
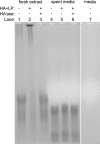
Understanding and controlling chondrocyte and cartilage metabolism in osteochondral tissues may facilitate ex vivo maintenance and application, both for allografts and tissue-engineered grafts. The hypothesis of this study was that maintenance of chondrocyte viability and matrix content and release of sulfated glycosaminoglycan (sGAG) in the articular cartilage of joint-scale osteochondral fragments are temperature and metabolism dependent. The aims were to assess, for adult goat joints, the effects of incubation temperature (37 degrees C vs. 4 degrees C) on cartilage chondrocyte viability and tissue matrix content and mechanical function, and the effects of temperature and cellular biosynthesis on sGAG release. Chondrocyte viability was maintained with 37 degrees C incubation for 28 days, but decreased by approximately 30% with 4 degrees C incubation. Concomitantly, with 37 degrees C incubation, cartilage sGAG was depleted by approximately 52% with the lost sGAG predominantly unable to aggregate with hyaluronan, whereas collagen content, tissue thickness, and tissue stiffness were maintained. The depletion of sGAG was diminished by slowing metabolism, with 4 degrees C decreasing release by approximately 79% compared with 37 degrees C incubation, and cycloheximide inhibition of cell metabolism at 37 degrees C decreasing release by approximately 47%. These results indicate that the articular cartilage of joint-scale grafts have enhanced chondrocyte viability with incubation at 37 degrees C, but may need anabolic stimuli or catabolic inhibitors to maintain sGAG content.
Figures


Similar articles
-
Comparison of the degradation of type II collagen and proteoglycan in nasal and articular cartilages induced by interleukin-1 and the selective inhibition of type II collagen cleavage by collagenase.Arthritis Rheum. 2000 Mar;43(3):664-72. doi: 10.1002/1529-0131(200003)43:3<664::AID-ANR24>3.0.CO;2-D. Arthritis Rheum. 2000. PMID: 10728761
-
Chondrocyte viability is higher after prolonged storage at 37 degrees C than at 4 degrees C for osteochondral grafts.Am J Sports Med. 2009 Nov;37 Suppl 1(Suppl 1):24S-32S. doi: 10.1177/0363546509351496. Epub 2009 Oct 27. Am J Sports Med. 2009. PMID: 19861697 Free PMC article.
-
Vulnerability of the superficial zone of immature articular cartilage to compressive injury.Arthritis Rheum. 2010 Oct;62(10):3016-27. doi: 10.1002/art.27610. Arthritis Rheum. 2010. PMID: 20556809 Free PMC article.
-
Cartilage storage at 4 °C with regular culture medium replacement benefits chondrocyte viability of osteochondral grafts in vitro.Cell Tissue Bank. 2016 Sep;17(3):473-9. doi: 10.1007/s10561-016-9556-7. Epub 2016 Apr 29. Cell Tissue Bank. 2016. PMID: 27130198 Free PMC article.
-
Effects of shear stress on articular chondrocyte metabolism.Biorheology. 2000;37(1-2):95-107. Biorheology. 2000. PMID: 10912182 Review.
Cited by
-
Altered swelling and ion fluxes in articular cartilage as a biomarker in osteoarthritis and joint immobilization: a computational analysis.J R Soc Interface. 2015 Jan 6;12(102):20141090. doi: 10.1098/rsif.2014.1090. J R Soc Interface. 2015. PMID: 25392400 Free PMC article.
-
Osteochondral allograft transplantation in cartilage repair: Graft storage paradigm, translational models, and clinical applications.J Orthop Res. 2016 Jan;34(1):31-8. doi: 10.1002/jor.22998. Epub 2015 Sep 24. J Orthop Res. 2016. PMID: 26234194 Free PMC article. Review.
-
Modernizing Storage Conditions for Fresh Osteochondral Allografts by Optimizing Viability at Physiologic Temperatures and Conditions.Cartilage. 2021 Dec;13(1_suppl):280S-292S. doi: 10.1177/1947603519888798. Epub 2019 Nov 28. Cartilage. 2021. PMID: 31777278 Free PMC article.
-
Recombinant human FGF18 preserves depth-dependent mechanical inhomogeneity in articular cartilage.Eur Cell Mater. 2019 Aug 8;38:23-34. doi: 10.22203/eCM.v038a03. Eur Cell Mater. 2019. PMID: 31393594 Free PMC article.
-
The in vivo performance of osteochondral allografts in the goat is diminished with extended storage and decreased cartilage cellularity.Am J Sports Med. 2012 Aug;40(8):1814-23. doi: 10.1177/0363546512449321. Epub 2012 Jun 15. Am J Sports Med. 2012. PMID: 22707746 Free PMC article.
References
-
- Gortz S. Bugbee W.D. Fresh osteochondral allografts: graft processing and clinical applications. J Knee Surg. 2006;19:231. - PubMed
-
- McNickle A.G. Provencher M.T. Cole B.J. Overview of existing cartilage repair technology. Sports Med Arthrosc. 2008;16:196. - PubMed
-
- Alford J.W. Cole B.J. Cartilage restoration, part 2: techniques, outcomes, and future directions. Am J Sports Med. 2005;33:443. - PubMed
-
- Hung C.T. Lima E.G. Mauck R.L. Taki E. LeRoux M.A. Lu H.H. Stark R.G. Guo X.E. Ateshian G.A. Anatomically shaped osteochondral constructs for articular cartilage repair. J Biomech. 2003;36:1853. - PubMed
-
- Isogai N. Landis W. Kim T.H. Gerstenfeld L.C. Upton J. Vacanti J.P. Formation of phalanges and small joints by tissue-engineering. J Bone Joint Surg Am. 1999;81-A:306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

