Divergence of allosteric effects of rapacuronium on binding and function of muscarinic receptors
- PMID: 20038295
- PMCID: PMC2806265
- DOI: 10.1186/1471-2210-9-15
Divergence of allosteric effects of rapacuronium on binding and function of muscarinic receptors
Abstract
Background: Many neuromuscular blockers act as negative allosteric modulators of muscarinic acetylcholine receptors by decreasing affinity and potency of acetylcholine. The neuromuscular blocker rapacuronium has been shown to have facilitatory effects at muscarinic receptors leading to bronchospasm. We examined the influence of rapacuronium on acetylcholine (ACh) binding to and activation of individual subtypes of muscarinic receptors expressed in Chinese hamster ovary cells to determine its receptor selectivity.
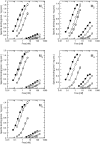
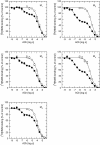
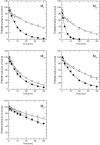
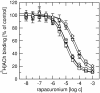
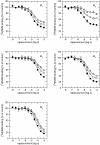
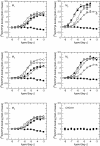
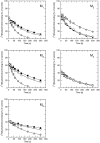
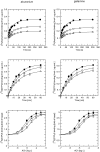
Results: At equilibrium rapacuronium bound to all subtypes of muscarinic receptors with micromolar affinity (2.7-17 microM) and displayed negative cooperativity with both high- and low-affinity ACh binding states. Rapacuronium accelerated [3H]ACh association with and dissociation from odd-numbered receptor subtypes. With respect to [35S]GTPgammaS binding rapacuronium alone behaved as an inverse agonist at all subtypes. Rapacuronium concentration-dependently decreased the potency of ACh-induced [35S]GTPgammaS binding at M2 and M4 receptors. In contrast, 0.1 microM rapacuronium significantly increased ACh potency at M1, M3, and M5 receptors. Kinetic measurements at M3 receptors showed acceleration of the rate of ACh-induced [35S]GTPgammaS binding by rapacuronium.
Conclusions: Our data demonstrate a novel dichotomy in rapacuronium effects at odd-numbered muscarinic receptors. Rapacuronium accelerates the rate of ACh binding but decreases its affinity under equilibrium conditions. This results in potentiation of receptor activation at low concentrations of rapacuronium (1 microM) but not at high concentrations (10 microM). These observations highlight the relevance and necessity of performing physiological tests under non-equilibrium conditions in evaluating the functional effects of allosteric modulators at muscarinic receptors. They also provide molecular basis for potentiating M3 receptor-mediated bronchoconstriction.
Figures


Similar articles
-
Allosteric effects of four stereoisomers of a fused indole ring system with 3H-N-methylscopolamine and acetylcholine at M1-M4 muscarinic receptors.Life Sci. 1999;64(6-7):519-26. doi: 10.1016/s0024-3205(98)00596-7. Life Sci. 1999. PMID: 10069518
-
A mechanism for rapacuronium-induced bronchospasm: M2 muscarinic receptor antagonism.Anesthesiology. 2003 Apr;98(4):906-11. doi: 10.1097/00000542-200304000-00017. Anesthesiology. 2003. PMID: 12657852
-
Rapacuronium augments acetylcholine-induced bronchoconstriction via positive allosteric interactions at the M3 muscarinic receptor.Anesthesiology. 2005 Dec;103(6):1195-203. doi: 10.1097/00000542-200512000-00014. Anesthesiology. 2005. PMID: 16306732
-
Selective allosteric enhancement of the binding and actions of acetylcholine at muscarinic receptor subtypes.Life Sci. 1997;60(13-14):1047-52. doi: 10.1016/s0024-3205(97)00046-5. Life Sci. 1997. PMID: 9121346 Review.
-
Allosteric activators of muscarinic receptors as novel approaches for treatment of CNS disorders.Mol Biosyst. 2010 Aug;6(8):1345-54. doi: 10.1039/c002938f. Epub 2010 Jun 25. Mol Biosyst. 2010. PMID: 20582339 Free PMC article. Review.
Cited by
-
Allosteric Modulation of Muscarinic Acetylcholine Receptors.Pharmaceuticals (Basel). 2010 Aug 30;3(9):2838-2860. doi: 10.3390/ph3092838. Pharmaceuticals (Basel). 2010. PMID: 27713379 Free PMC article. Review.
-
Catestatin attenuates endoplasmic reticulum induced cell apoptosis by activation type 2 muscarinic acetylcholine receptor in cardiac ischemia/reperfusion.Sci Rep. 2015 Nov 16;5:16590. doi: 10.1038/srep16590. Sci Rep. 2015. PMID: 26567709 Free PMC article.
References
-
- Caulfield MP, Birdsall NJ. International union of pharmacology. XVII. classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998;50:279–290. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

