Immunization with a combination of three pneumococcal proteins confers additive and broad protection against Streptococcus pneumoniae Infections in Mice
- PMID: 20038538
- PMCID: PMC2825939
- DOI: 10.1128/IAI.00473-09
Immunization with a combination of three pneumococcal proteins confers additive and broad protection against Streptococcus pneumoniae Infections in Mice
Abstract
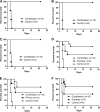
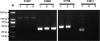
Pneumococcal polysaccharide-based vaccines are effective in preventing pneumococcus infection; however, some drawbacks preclude their widespread use in developing and undeveloped countries. Here, we evaluated the protective effects of ATP-dependent caseinolytic protease (ClpP), pneumolysin mutant (DeltaA146 Ply), putative lipoate-protein ligase (Lpl), or combinations thereof against pneumococcal infections in mice. Vaccinated mice were intraperitoneally and/or intranasally challenged with different pneumococcal strains. In intraperitoneal challenge models with pneumococcal strain D39 (serotype 2), the most striking protection was obtained with the combination of the three antigens. Similarly, with the intranasal challenge models, (i) additive clearance of bacteria in lungs was observed for the combination of the three antigens and (ii) a combination vaccine conferred complete protection against intranasal infections of three of the four most common pneumococcal strains (serotypes 14, 19F, and 23F) and 80% protection for pneumococcal strain 6B. Even so, immunity to this combination could confer protection against pneumococcal infection with a mixture of four serotypes. Our results showed that the combination vaccine was as effective as the currently used vaccines (PCV7 and PPV23). These results indicate that system immunization with the combination of pneumococcal antigens could provide an additive and broad protection against Streptococcus pneumoniae in pneumonia and sepsis infection models.
Figures
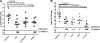
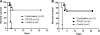
References
-
- Alexander, J. E., R. A. Lock, C. C. A. M. Peeters, J. T. Poolman, P. W. Andrew, T. J. Mitchell, D. Hansman, and J. C. Paton. 1994. Immunization of mice with pneumolysin toxoid confers a significant degree of protection against at least nine serotypes of Streptococcus pneumoniae. Infect. Immun. 62:5683-5688. - PMC - PubMed
-
- Aslan, G., G. Emekdas, M. Bayer, M. S. Serin, N. Kuyucu, and A. Kanik. 2007. Serotype distribution of Streptococcus pneumoniae strains in the nasopharynx of healthy Turkish children. Indian J. Med. Res. 125:582-587. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

