Constitutively active inflammasome in human melanoma cells mediating autoinflammation via caspase-1 processing and secretion of interleukin-1beta
- PMID: 20038581
- PMCID: PMC2825443
- DOI: 10.1074/jbc.M109.064907
Constitutively active inflammasome in human melanoma cells mediating autoinflammation via caspase-1 processing and secretion of interleukin-1beta
Abstract
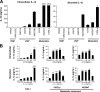
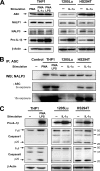
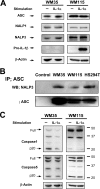
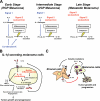
Interleukin-1beta (IL-1beta) is a pleiotropic cytokine promoting inflammation, angiogenesis, and tissue remodeling as well as regulation of immune responses. Although IL-1beta contributes to growth and metastatic spread in experimental and human cancers, the molecular mechanisms regulating the conversion of the inactive IL-1beta precursor to a secreted and active cytokine remains unclear. Here we demonstrate that NALP3 inflammasome is constitutively assembled and activated with cleavage of caspase-1 in human melanoma cells. Late stage human melanoma cells spontaneously secrete active IL-1beta via constitutive activation of the NALP3 inflammasome and IL-1 receptor signaling, exhibiting a feature of autoinflammatory diseases. Unlike human blood monocytes, these melanoma cells require no exogenous stimulation. In contrast, NALP3 functionality in intermediate stage melanoma cells requires activation of the IL-1 receptor to secrete active IL-1beta; cells from an early stage of melanoma require stimulation of the IL-1 receptor plus the co-stimulant muramyl dipeptide. The spontaneous secretion of IL-1beta from melanoma cells was reduced by inhibition of caspase-1 or the use of small interfering RNA directed against ASC. Supernatants from melanoma cell cultures enhanced macrophage chemotaxis and promoted in vitro angiogenesis, both prevented by pretreating melanoma cells with inhibitors of caspases-1 and -5 or IL-1 receptor blockade. These findings implicate IL-1-mediated autoinflammation as contributing to the development and progression of human melanoma and suggest that inhibiting the inflammasome pathway or reducing IL-1 activity can be a therapeutic option for melanoma patients.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

