Coproporphyrin excretion and low thiol levels caused by point mutation in the Rhodobacter sphaeroides S-adenosylmethionine synthetase gene
- PMID: 20038586
- PMCID: PMC2820862
- DOI: 10.1128/JB.01342-09
Coproporphyrin excretion and low thiol levels caused by point mutation in the Rhodobacter sphaeroides S-adenosylmethionine synthetase gene
Abstract
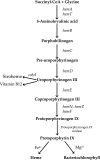
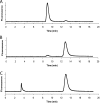
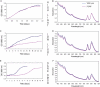
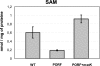
A spontaneous mutant of Rhodobacter sphaeroides f. sp. denitrificans IL-106 was found to excrete a large amount of a red compound identified as coproporphyrin III, an intermediate in bacteriochlorophyll and heme synthesis. The mutant, named PORF, is able to grow under phototrophic conditions but has low levels of intracellular cysteine and glutathione and overexpresses the cysteine synthase CysK. The expression of molybdoenzymes such as dimethyl sulfoxide (DMSO) and nitrate reductases is also affected under certain growth conditions. Excretion of coproporphyrin and overexpression of CysK are not directly related but were both found to be consequences of a diminished synthesis of the key metabolite S-adenosylmethionine (SAM). The wild-type phenotype is restored when the gene metK encoding SAM synthetase is supplied in trans. The metK gene in the mutant strain has a mutation leading to a single amino acid change (H145Y) in the encoded protein. This point mutation is responsible for a 70% decrease in intracellular SAM content which probably affects the activities of numerous SAM-dependent enzymes such as coproporphyrinogen oxidase (HemN); uroporphyrinogen III methyltransferase (CobA), which is involved in siroheme synthesis; and molybdenum cofactor biosynthesis protein A (MoaA). We propose a model showing that the attenuation of the activities of SAM-dependent enzymes in the mutant could be responsible for the coproporphyrin excretion, the low cysteine and glutathione contents, and the decrease in DMSO and nitrate reductase activities.
Figures


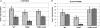


Similar articles
-
Identification of a mutation in the Bacillus subtilis S-adenosylmethionine synthetase gene that results in derepression of S-box gene expression.J Bacteriol. 2006 May;188(10):3674-81. doi: 10.1128/JB.188.10.3674-3681.2006. J Bacteriol. 2006. PMID: 16672621 Free PMC article.
-
Enhanced expression of S-adenosylmethionine synthetase causes overproduction of actinorhodin in Streptomyces coelicolor A3(2).J Bacteriol. 2003 Jan;185(2):601-9. doi: 10.1128/JB.185.2.601-609.2003. J Bacteriol. 2003. PMID: 12511507 Free PMC article.
-
Cloning and nucleotide sequence of the gene encoding dimethyl sulfoxide reductase from Rhodobacter sphaeroides f. sp. denitrificans.Biosci Biotechnol Biochem. 1995 Oct;59(10):1850-5. doi: 10.1271/bbb.59.1850. Biosci Biotechnol Biochem. 1995. PMID: 8534974
-
Pleiotropic effects of methionine adenosyltransferases deregulation as determinants of liver cancer progression and prognosis.J Hepatol. 2013 Oct;59(4):830-41. doi: 10.1016/j.jhep.2013.04.031. Epub 2013 May 7. J Hepatol. 2013. PMID: 23665184 Review.
-
Methionine adenosyltransferase I/III deficiency: neurological manifestations and relevance of S-adenosylmethionine.Mol Genet Metab. 2012 Nov;107(3):253-6. doi: 10.1016/j.ymgme.2012.08.002. Epub 2012 Aug 11. Mol Genet Metab. 2012. PMID: 22951388 Review.
Cited by
-
Gene expression profiling in Entamoeba histolytica identifies key components in iron uptake and metabolism.PLoS One. 2014 Sep 11;9(9):e107102. doi: 10.1371/journal.pone.0107102. eCollection 2014. PLoS One. 2014. PMID: 25210888 Free PMC article.
-
Detrimental effect of the 6 His C-terminal tag on YedY enzymatic activity and influence of the TAT signal sequence on YedY synthesis.BMC Biochem. 2013 Nov 1;14:28. doi: 10.1186/1471-2091-14-28. BMC Biochem. 2013. PMID: 24180491 Free PMC article.
-
Porphyrin Excretion Resulting From Mutation of a Gene Encoding a Class I Fructose 1,6-Bisphosphate Aldolase in Rhodobacter capsulatus.Front Microbiol. 2019 Feb 22;10:301. doi: 10.3389/fmicb.2019.00301. eCollection 2019. Front Microbiol. 2019. PMID: 30853951 Free PMC article.
-
Detection and Sequencing of Iron Superoxide Dismutase Gene in Entamoeba histolytica Isolated from Patients with Diarrhea in Iraq.Arch Razi Inst. 2021 Nov 30;76(5):1289-1295. doi: 10.22092/ari.2021.356101.1777. eCollection 2021 Nov. Arch Razi Inst. 2021. PMID: 35355778 Free PMC article.
References
-
- Arnoux, P., M. Sabaty, J. Alric, B. Frangioni, B. Guigliarelli, J. M. Adriano, and D. Pignol. 2003. Structural and redox plasticity in the heterodimeric periplasmic nitrate reductase. Nat. Struct. Biol. 10:928-934. - PubMed
-
- Brokx, S. J., R. A. Rothery, G. Zhang, D. P. Ng, and J. H. Weiner. 2005. Characterization of an Escherichia coli sulfite oxidase homologue reveals the role of a conserved active site cysteine in assembly and function. Biochemistry 44:10339-10348. - PubMed
-
- Cantoni, G. L. 1975. Biological methylation: selected aspects. Annu. Rev. Biochem. 44:435-451. - PubMed
-
- Chartrand, P., D. Tardif, and A. Sasarman. 1979. Uroporphyrin- and coproporphyrin I-accumulating mutant of Escherichia coli K12. J. Gen. Microbiol. 110:61-66. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources

