Decoration of T-independent antigen with ligands for CD22 and Siglec-G can suppress immunity and induce B cell tolerance in vivo
- PMID: 20038598
- PMCID: PMC2812539
- DOI: 10.1084/jem.20091873
Decoration of T-independent antigen with ligands for CD22 and Siglec-G can suppress immunity and induce B cell tolerance in vivo
Erratum in
- J Exp Med. 2010 Feb 15;207(2):445. Paulson, James [corrected to Paulson, James C]
Abstract
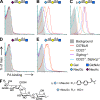
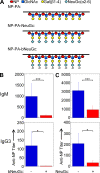
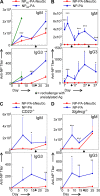
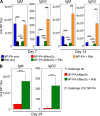
Autoreactive B lymphocytes first encountering self-antigens in peripheral tissues are normally regulated by induction of anergy or apoptosis. According to the "two-signal" model, antigen recognition alone should render B cells tolerant unless T cell help or inflammatory signals such as lipopolysaccharide are provided. However, no such signals seem necessary for responses to T-independent type 2 (TI-2) antigens, which are multimeric antigens lacking T cell epitopes and Toll-like receptor ligands. How then do mature B cells avoid making a TI-2-like response to multimeric self-antigens? We present evidence that TI-2 antigens decorated with ligands of inhibitory sialic acid-binding Ig-like lectins (siglecs) are poorly immunogenic and can induce tolerance to subsequent challenge with immunogenic antigen. Two siglecs, CD22 and Siglec-G, contributed to tolerance induction, preventing plasma cell differentiation or survival. Although mutations in CD22 and its signaling machinery have been associated with dysregulated B cell development and autoantibody production, previous analyses failed to identify a tolerance defect in antigen-specific mutant B cells. Our results support a role for siglecs in B cell self-/nonself-discrimination, namely suppressing responses to self-associated antigens while permitting rapid "missing self"-responses to unsialylated multimeric antigens. The results suggest use of siglec ligand antigen constructs as an approach for inducing tolerance.
Figures




References
-
- Blixt O., Collins B.E., van den Nieuwenhof I.M., Crocker P.R., Paulson J.C. 2003. Sialoside specificity of the siglec family assessed using novel multivalent probes: identification of potent inhibitors of myelin-associated glycoprotein. J. Biol. Chem. 278:31007–31019 doi:10.1074/jbc.M304331200 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

