CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells
- PMID: 20038600
- PMCID: PMC2812532
- DOI: 10.1084/jem.20091964
CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells
Erratum in
- J Exp Med. 2010 Feb 15;207(2):447
Abstract
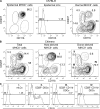
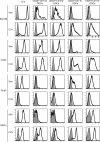
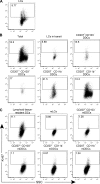
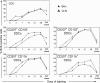
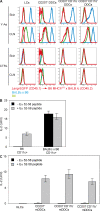
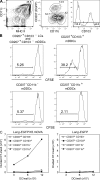
Recent studies have challenged the view that Langerhans cells (LCs) constitute the exclusive antigen-presenting cells of the skin and suggest that the dermal dendritic cell (DDC) network is exceedingly complex. Using knockin mice to track and ablate DCs expressing langerin (CD207), we discovered that the dermis contains five distinct DC subsets and identified their migratory counterparts in draining lymph nodes. Based on this refined classification, we demonstrated that the quantitatively minor CD207+ CD103+ DDC subset is endowed with the unique capability of cross-presenting antigens expressed by keratinocytes irrespective of the presence of LCs. We further showed that Y-Ae, an antibody that is widely used to monitor the formation of complexes involving I-Ab molecules and a peptide derived from the I-E alpha chain, recognizes mature skin DCs that express I-Ab molecules in the absence of I-E alpha. Knowledge of this extra reactivity is important because it could be, and already has been, mistakenly interpreted to support the view that antigen transfer can occur between LCs and DDCs. Collectively, these data revisit the transfer of antigen that occurs between keratinocytes and the five distinguishable skin DC subsets and stress the high degree of functional specialization that exists among them.
Figures


References
-
- Bennett C.L., Noordegraaf M., Martina C.A., Clausen B.E. 2007. Langerhans cells are required for efficient presentation of topically applied hapten to T cells. J. Immunol. 179:6830–6835 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

