Essential role of mannose-binding lectin-associated serine protease-1 in activation of the complement factor D
- PMID: 20038603
- PMCID: PMC2812541
- DOI: 10.1084/jem.20090633
Essential role of mannose-binding lectin-associated serine protease-1 in activation of the complement factor D
Abstract
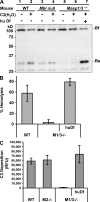
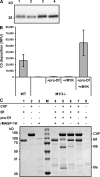
The complement system is an essential component of innate immunity, participating in the pathogenesis of inflammatory diseases and in host defense. In the lectin complement pathway, mannose-binding lectin (MBL) and ficolins act as recognition molecules, and MBL-associated serine protease (MASP) is a key enzyme; MASP-2 is responsible for the lectin pathway activation. The function of other serine proteases (MASP-1 and MASP-3) is still obscure. In this study, we generated a MASP-1- and MASP-3-deficient mouse model (Masp1/3-/-) and found that no activation of the alternative pathway was observed in Masp1/3-/- serum. Mass spectrometric analysis revealed that circulating complement factor D (Df) in Masp1/3-/- mice is a zymogen (pro-Df) with the activation peptide QPRGR at its N terminus. These results suggested that Masp1/3-/- mice failed to convert pro-Df to its active form, whereas it was generally accepted that the activation peptide of pro-Df is removed during its secretion and factor D constitutively exists in an active form in the circulation. Furthermore, recombinant MASP-1 converted pro-Df to the active form in vitro, although the activation mechanism of pro-Df by MASP-1 is still unclear. Thus, it is clear that MASP-1 is an essential protease of both the lectin and alternative complement pathways.
Figures



Similar articles
-
Mannan-Binding Lectin-Associated Serine Protease 1/3 Cleavage of Pro-Factor D into Factor D In Vivo and Attenuation of Collagen Antibody-Induced Arthritis through Their Targeted Inhibition by RNA Interference-Mediated Gene Silencing.J Immunol. 2016 Nov 1;197(9):3680-3694. doi: 10.4049/jimmunol.1600719. Epub 2016 Oct 5. J Immunol. 2016. PMID: 27707997 Free PMC article.
-
Mannose-binding lectin (MBL)-associated serine protease (MASP)-1 contributes to activation of the lectin complement pathway.J Immunol. 2008 May 1;180(9):6132-8. doi: 10.4049/jimmunol.180.9.6132. J Immunol. 2008. PMID: 18424734
-
Essential Roles for Mannose-Binding Lectin-Associated Serine Protease-1/3 in the Development of Lupus-Like Glomerulonephritis in MRL/lpr Mice.Front Immunol. 2018 May 28;9:1191. doi: 10.3389/fimmu.2018.01191. eCollection 2018. Front Immunol. 2018. PMID: 29892304 Free PMC article.
-
Factor D.Immunol Rev. 2023 Jan;313(1):15-24. doi: 10.1111/imr.13155. Epub 2022 Oct 31. Immunol Rev. 2023. PMID: 36316810 Review.
-
The role of MASP-1/3 in complement activation.Adv Exp Med Biol. 2013;735:41-53. doi: 10.1007/978-1-4614-4118-2_3. Adv Exp Med Biol. 2013. PMID: 23402018 Review.
Cited by
-
Effects of MASP-1 of the complement system on activation of coagulation factors and plasma clot formation.PLoS One. 2012;7(4):e35690. doi: 10.1371/journal.pone.0035690. Epub 2012 Apr 20. PLoS One. 2012. PMID: 22536427 Free PMC article.
-
Mannan-Binding Lectin-Associated Serine Protease 1/3 Cleavage of Pro-Factor D into Factor D In Vivo and Attenuation of Collagen Antibody-Induced Arthritis through Their Targeted Inhibition by RNA Interference-Mediated Gene Silencing.J Immunol. 2016 Nov 1;197(9):3680-3694. doi: 10.4049/jimmunol.1600719. Epub 2016 Oct 5. J Immunol. 2016. PMID: 27707997 Free PMC article.
-
Key Components of the Complement Lectin Pathway Are Not Only Required for the Development of Inflammatory Arthritis but Also Regulate the Transcription of Factor D.Front Immunol. 2020 Feb 21;11:201. doi: 10.3389/fimmu.2020.00201. eCollection 2020. Front Immunol. 2020. PMID: 32153567 Free PMC article.
-
Mannose-binding lectin and the balance between immune protection and complication.Expert Rev Anti Infect Ther. 2011 Dec;9(12):1179-90. doi: 10.1586/eri.11.136. Expert Rev Anti Infect Ther. 2011. PMID: 22114968 Free PMC article. Review.
-
Mannan binding lectin-associated serine protease-2 (MASP-2) critically contributes to post-ischemic brain injury independent of MASP-1.J Neuroinflammation. 2016 Aug 30;13(1):213. doi: 10.1186/s12974-016-0684-6. J Neuroinflammation. 2016. PMID: 27577570 Free PMC article.
References
-
- Ambrus G., Gál P., Kojima M., Szilágyi K., Balczer J., Antal J., Gráf L., Laich A., Moffatt B.E., Schwaeble W., et al. 2003. Natural substrates and inhibitors of mannan-binding lectin-associated serine protease-1 and -2: a study on recombinant catalytic fragments. J. Immunol. 170:1374–1382 - PubMed
-
- Barnum S.R., Volanakis J.E. 1985. In vitro biosynthesis of complement protein D by U937 cells. J. Immunol. 134:1799–1803 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

