Yeast cell adhesion molecules have functional amyloid-forming sequences
- PMID: 20038605
- PMCID: PMC2837979
- DOI: 10.1128/EC.00068-09
Yeast cell adhesion molecules have functional amyloid-forming sequences
Abstract
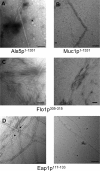
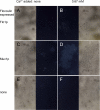
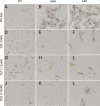
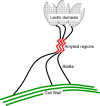
The occurrence of highly conserved amyloid-forming sequences in Candida albicans Als proteins (H. N. Otoo et al., Eukaryot. Cell 7:776-782, 2008) led us to search for similar sequences in other adhesins from C. albicans and Saccharomyces cerevisiae. The beta-aggregation predictor TANGO found highly beta-aggregation-prone sequences in almost all yeast adhesins. These sequences had an unusual amino acid composition: 77% of their residues were beta-branched aliphatic amino acids Ile, Thr, and Val, which is more than 4-fold greater than their prevalence in the S. cerevisiae proteome. High beta-aggregation potential peptides from S. cerevisiae Flo1p and C. albicans Eap1p rapidly formed insoluble amyloids, as determined by Congo red absorbance, thioflavin T fluorescence, and fiber morphology. As examples of the amyloid-forming ability of the native proteins, soluble glycosylphosphatidylinositol (GPI)-less fragments of C. albicans Als5p and S. cerevisiae Muc1p also formed amyloids within a few days under native conditions at nM concentrations. There was also evidence of amyloid formation in vivo: the surfaces of cells expressing wall-bound Als1p, Als5p, Muc1p, or Flo1p were birefringent and bound the fluorescent amyloid-reporting dye thioflavin T. Both of these properties increased upon aggregation of the cells. In addition, amyloid binding dyes strongly inhibited aggregation and flocculation. The results imply that amyloid formation is an intrinsic property of yeast cell adhesion proteins from many gene families and that amyloid formation is an important component of cellular aggregation mediated by these proteins.
Figures




Similar articles
-
An Amyloid Core Sequence in the Major Candida albicans Adhesin Als1p Mediates Cell-Cell Adhesion.mBio. 2019 Oct 8;10(5):e01766-19. doi: 10.1128/mBio.01766-19. mBio. 2019. PMID: 31594814 Free PMC article.
-
Candida albicans Als adhesins have conserved amyloid-forming sequences.Eukaryot Cell. 2008 May;7(5):776-82. doi: 10.1128/EC.00309-07. Epub 2007 Dec 14. Eukaryot Cell. 2008. PMID: 18083824 Free PMC article.
-
A role for amyloid in cell aggregation and biofilm formation.PLoS One. 2011 Mar 8;6(3):e17632. doi: 10.1371/journal.pone.0017632. PLoS One. 2011. PMID: 21408122 Free PMC article.
-
Amyloid-Like β-Aggregates as Force-Sensitive Switches in Fungal Biofilms and Infections.Microbiol Mol Biol Rev. 2017 Nov 29;82(1):e00035-17. doi: 10.1128/MMBR.00035-17. Print 2018 Mar. Microbiol Mol Biol Rev. 2017. PMID: 29187516 Free PMC article. Review.
-
Adhesion in Candida spp.Cell Microbiol. 2002 Aug;4(8):461-9. doi: 10.1046/j.1462-5822.2002.00206.x. Cell Microbiol. 2002. PMID: 12174081 Review.
Cited by
-
Peptide detection of fungal functional amyloids in infected tissue.PLoS One. 2014 Jan 21;9(1):e86067. doi: 10.1371/journal.pone.0086067. eCollection 2014. PLoS One. 2014. PMID: 24465872 Free PMC article.
-
Efg1 Controls caspofungin-induced cell aggregation of Candida albicans through the adhesin Als1.Eukaryot Cell. 2011 Dec;10(12):1694-704. doi: 10.1128/EC.05187-11. Epub 2011 Oct 28. Eukaryot Cell. 2011. PMID: 22037180 Free PMC article.
-
Cell Adhesion on Amyloid Fibrils Lacking Integrin Recognition Motif.J Biol Chem. 2016 Mar 4;291(10):5278-98. doi: 10.1074/jbc.M115.678177. Epub 2016 Jan 7. J Biol Chem. 2016. PMID: 26742841 Free PMC article.
-
An Amyloid Core Sequence in the Major Candida albicans Adhesin Als1p Mediates Cell-Cell Adhesion.mBio. 2019 Oct 8;10(5):e01766-19. doi: 10.1128/mBio.01766-19. mBio. 2019. PMID: 31594814 Free PMC article.
-
Role of force-sensitive amyloid-like interactions in fungal catch bonding and biofilms.Eukaryot Cell. 2014 Sep;13(9):1136-42. doi: 10.1128/EC.00068-14. Epub 2014 Mar 28. Eukaryot Cell. 2014. PMID: 24681687 Free PMC article.
References
-
- Aimanianda V., Bayry J., Bozza S., Kniemeyer O., Perruccio K., Elluru S. R., Clavaud C., Paris S., Brakhage A. A., Kaveri S. V., Romani L., Latge J. P. 2009. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 460:1117–1121 - PubMed
-
- Barten D. M., Albright C. F. 2008. Therapeutic strategies for Alzheimer's disease. Mol. Neurobiol. 37:171–186 - PubMed
-
- Baxa U., Cassese T., Kajava A. V., Steven A. C. 2006. Structure, function, and amyloidogenesis of fungal prions: filament polymorphism and prion variants. Adv. Protein Chem. 73:125–180 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

