Lack of pharmacokinetic interaction between amdoxovir and reduced- and standard-dose zidovudine in HIV-1-infected individuals
- PMID: 20038617
- PMCID: PMC2826005
- DOI: 10.1128/AAC.01209-09
Lack of pharmacokinetic interaction between amdoxovir and reduced- and standard-dose zidovudine in HIV-1-infected individuals
Abstract
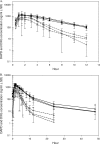
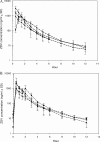
Amdoxovir (AMDX) inhibits HIV-1 containing the M184V/I mutation and is rapidly absorbed and deaminated to its active metabolite, beta-D-dioxolane guanosine (DXG). DXG is synergistic with zidovudine (ZDV) in HIV-1-infected primary human lymphocytes. A recent in silico pharmacokinetic (PK)/enzyme kinetic study suggested that ZDV at 200 mg twice a day (b.i.d.) may reduce toxicity without compromising efficacy relative to the standard 300-mg b.i.d. dose. Therefore, an intense PK clinical study was conducted using AMDX/placebo, with or without ZDV, in 24 subjects randomized to receive oral AMDX at 500 mg b.i.d., AMDX at 500 mg plus ZDV at 200 or 300 mg b.i.d., or ZDV at 200 or 300 mg b.i.d. for 10 days. Full plasma PK profiles were collected on days 1 and 10, and complete urine sampling was performed on day 9. Plasma and urine concentrations of AMDX, DXG, ZDV, and ZDV-5'-O-glucuronide (GZDV) were measured using a validated liquid chromatography-tandem mass spectrometry method. Data were analyzed using noncompartmental methods, and multiple comparisons were performed on the log-transformed parameters, at steady state. Coadministration of AMDX with ZDV did not significantly change either of the plasma PK parameters or percent recovery in the urine of AMDX, DXG, or ZDV/GZDV. Larger studies with AMDX/ZDV, with a longer duration, are warranted.
Figures
Similar articles
-
Antiviral activity and tolerability of amdoxovir with zidovudine in a randomized double-blind placebo-controlled study in HIV-1-infected individuals.Antivir Ther. 2010;15(2):185-92. doi: 10.3851/IMP1514. Antivir Ther. 2010. PMID: 20386073 Free PMC article. Clinical Trial.
-
Development of an optimized dose for coformulation of zidovudine with drugs that select for the K65R mutation using a population pharmacokinetic and enzyme kinetic simulation model.Antimicrob Agents Chemother. 2008 Dec;52(12):4241-50. doi: 10.1128/AAC.00054-08. Epub 2008 Oct 6. Antimicrob Agents Chemother. 2008. PMID: 18838591 Free PMC article.
-
Single-dose pharmacokinetics and safety of abacavir (1592U89), zidovudine, and lamivudine administered alone and in combination in adults with human immunodeficiency virus infection.Antimicrob Agents Chemother. 1999 Jul;43(7):1708-15. doi: 10.1128/AAC.43.7.1708. Antimicrob Agents Chemother. 1999. PMID: 10390227 Free PMC article. Clinical Trial.
-
Dioxolane guanosine, the active form of the prodrug diaminopurine dioxolane, is a potent inhibitor of drug-resistant HIV-1 isolates from patients for whom standard nucleoside therapy fails.J Acquir Immune Defic Syndr. 2002 Jan 1;29(1):11-20. doi: 10.1097/00042560-200201010-00002. J Acquir Immune Defic Syndr. 2002. PMID: 11782585
-
Intracellular studies of the nucleoside reverse transcriptase inhibitor active metabolites: a review.P R Health Sci J. 2000 Mar;19(1):19-27. P R Health Sci J. 2000. PMID: 10761201 Review.
Cited by
-
Practical Considerations For Developing Nucleoside Reverse Transcriptase Inhibitors.Drug Discov Today Technol. 2012 Fall;9(3):e183-e193. doi: 10.1016/j.ddtec.2012.09.003. Drug Discov Today Technol. 2012. PMID: 23554824 Free PMC article.
-
Scaleable processes for the synthesis of (-)-β-D-2,6-diaminopurine dioxolane (Amdoxovir, DAPD) and (-)-β-D-2-aminopurine dioxolane (APD).Tetrahedron. 2012 Jul 22;68(29):5738-5743. doi: 10.1016/j.tet.2012.05.039. Epub 2012 May 16. Tetrahedron. 2012. PMID: 23162170 Free PMC article.
-
Prodrug strategies for improved efficacy of nucleoside antiviral inhibitors.Curr Opin HIV AIDS. 2013 Nov;8(6):556-64. doi: 10.1097/COH.0000000000000007. Curr Opin HIV AIDS. 2013. PMID: 24100876 Free PMC article. Review.
-
Cellular pharmacology and potency of HIV-1 nucleoside analogs in primary human macrophages.Antimicrob Agents Chemother. 2013 Mar;57(3):1262-9. doi: 10.1128/AAC.02012-12. Epub 2012 Dec 21. Antimicrob Agents Chemother. 2013. PMID: 23263005 Free PMC article.
-
Antiviral activity and tolerability of amdoxovir with zidovudine in a randomized double-blind placebo-controlled study in HIV-1-infected individuals.Antivir Ther. 2010;15(2):185-92. doi: 10.3851/IMP1514. Antivir Ther. 2010. PMID: 20386073 Free PMC article. Clinical Trial.
References
-
- Acosta, E. P., L. M. Page, and C. V. Fletcher. 1996. Clinical pharmacokinetics of zidovudine. An update. Clin. Pharmacokinet. 30:251-262. - PubMed
-
- Barry, M. G., S. H. Khoo, G. J. Veal, P. G. Hoggard, S. E. Gibbons, E. G. Wilkins, O. Williams, A. M. Breckenridge, and D. J. Back. 1996. The effect of zidovudine dose on the formation of intracellular phosphorylated metabolites. AIDS 10:1361-1367. - PubMed
-
- Carpenter, C. C., D. A. Cooper, M. A. Fischl, J. M. Gatell, B. G. Gazzard, S. M. Hammer, M. S. Hirsch, D. M. Jacobsen, D. A. Katzenstein, J. S. Montaner, D. D. Richman, M. S. Saag, M. Schechter, R. T. Schooley, M. A. Thompson, S. Vella, P. G. Yeni, and P. A. Volberding. 2000. Antiretroviral therapy in adults: updated recommendations of the International AIDS Society—USA Panel. JAMA 283:381-390. - PubMed
-
- Chen, H., R. F. Schinazi, P. Rajagopalan, Z. Gao, C. K. Chu, H. M. McClure, and F. D. Boudinot. 1999. Pharmacokinetics of (−)-beta-d-dioxolane guanine and prodrug (−)-beta-d-2,6-diaminopurine dioxolane in rats and monkeys. AIDS Res. Hum. Retroviruses 15:1625-1630. - PubMed
-
- Chin, R., T. Shaw, J. Torresi, V. Sozzi, C. Trautwein, T. Bock, M. Manns, H. Isom, P. Furman, and S. Locarnini. 2001. In vitro susceptibilities of wild-type or drug-resistant hepatitis B virus to (−)-beta-d-2,6-diaminopurine dioxolane and 2′-fluoro-5-methyl-beta-l-arabinofuranosyluracil. Antimicrob. Agents Chemother. 45:2495-2501. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

