Opposite-polarity motors activate one another to trigger cargo transport in live cells
- PMID: 20038680
- PMCID: PMC2806283
- DOI: 10.1083/jcb.200908075
Opposite-polarity motors activate one another to trigger cargo transport in live cells
Abstract
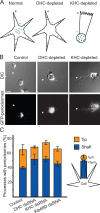
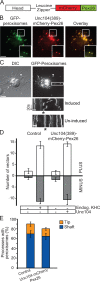
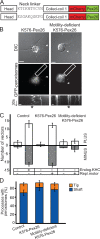
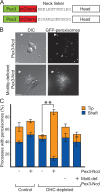
Intracellular transport is typically bidirectional, consisting of a series of back and forth movements. Kinesin-1 and cytoplasmic dynein require each other for bidirectional transport of intracellular cargo along microtubules; i.e., inhibition or depletion of kinesin-1 abolishes dynein-driven cargo transport and vice versa. Using Drosophila melanogaster S2 cells, we demonstrate that replacement of endogenous kinesin-1 or dynein with an unrelated, peroxisome-targeted motor of the same directionality activates peroxisome transport in the opposite direction. However, motility-deficient versions of motors, which retain the ability to bind microtubules and hydrolyze adenosine triphosphate, do not activate peroxisome motility. Thus, any pair of opposite-polarity motors, provided they move along microtubules, can activate one another. These results demonstrate that mechanical interactions between opposite-polarity motors are necessary and sufficient for bidirectional organelle transport in live cells.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

