Assessing niche separation among coexisting Limnohabitans strains through interactions with a competitor, viruses, and a bacterivore
- PMID: 20038688
- PMCID: PMC2832377
- DOI: 10.1128/AEM.02517-09
Assessing niche separation among coexisting Limnohabitans strains through interactions with a competitor, viruses, and a bacterivore
Erratum in
- Appl Environ Microbiol. 2010 Jun;76(11):3762
Abstract
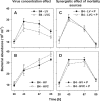
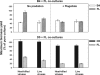
We investigated potential niche separation in two closely related (99.1% 16S rRNA gene sequence similarity) syntopic bacterial strains affiliated with the R-BT065 cluster, which represents a subgroup of the genus Limnohabitans. The two strains, designated B4 and D5, were isolated concurrently from a freshwater reservoir. Differences between the strains were examined through monitoring interactions with a bacterial competitor, Flectobacillus sp. (FL), and virus- and predator-induced mortality. Batch-type cocultures, designated B4+FL and D5+FL, were initiated with a similar biomass ratio among the strains. The proportion of each cell type present in the cocultures was monitored based on clear differences in cell sizes. Following exponential growth for 28 h, the cocultures were amended by the addition of two different concentrations of live or heat-inactivated viruses concentrated from the reservoir. Half of virus-amended treatments were inoculated immediately with an axenic flagellate predator, Poterioochromonas sp. The presence of the predator, of live viruses, and of competition between the strains significantly affected their population dynamics in the experimentally manipulated treatments. While strains B4 and FL appeared vulnerable to environmental viruses, strain D5 did not. Predator-induced mortality had the greatest impact on FL, followed by that on D5 and then B4. The virus-vulnerable B4 strain had smaller cells and lower biomass yield, but it was less subject to grazing. In contrast, the seemingly virus-resistant D5, with slightly larger grazing-vulnerable cells, was competitive with FL. Overall, our data suggest contrasting ecophysiological capabilities and partial niche separation in two coexisting Limnohabitans strains.
Figures





Similar articles
-
Prey-Specific Growth Responses of Freshwater Flagellate Communities Induced by Morphologically Distinct Bacteria from the Genus Limnohabitans.Appl Environ Microbiol. 2015 Aug;81(15):4993-5002. doi: 10.1128/AEM.00396-15. Epub 2015 May 15. Appl Environ Microbiol. 2015. PMID: 25979896 Free PMC article.
-
Aggregate formation in a freshwater bacterial strain induced by growth state and conspecific chemical cues.Environ Microbiol. 2010 Sep;12(9):2486-95. doi: 10.1111/j.1462-2920.2010.02222.x. Epub 2010 Apr 19. Environ Microbiol. 2010. PMID: 20406293
-
Interspecific competition and protistan grazing affect the coexistence of freshwater betaproteobacterial strains.FEMS Microbiol Ecol. 2016 Feb;92(2):fiv156. doi: 10.1093/femsec/fiv156. Epub 2015 Dec 9. FEMS Microbiol Ecol. 2016. PMID: 26656063
-
Predation on prokaryotes in the water column and its ecological implications.Nat Rev Microbiol. 2005 Jul;3(7):537-46. doi: 10.1038/nrmicro1180. Nat Rev Microbiol. 2005. PMID: 15953930 Review.
-
Competition and niche separation of pelagic bacteria in freshwater habitats.Environ Microbiol. 2017 Jun;19(6):2133-2150. doi: 10.1111/1462-2920.13742. Epub 2017 May 10. Environ Microbiol. 2017. PMID: 28370850 Review.
Cited by
-
The diversity of the Limnohabitans genus, an important group of freshwater bacterioplankton, by characterization of 35 isolated strains.PLoS One. 2013;8(3):e58209. doi: 10.1371/journal.pone.0058209. Epub 2013 Mar 7. PLoS One. 2013. PMID: 23505469 Free PMC article.
-
Temperature and Nutrient Levels Correspond with Lineage-Specific Microdiversification in the Ubiquitous and Abundant Freshwater Genus Limnohabitans.Appl Environ Microbiol. 2020 May 5;86(10):e00140-20. doi: 10.1128/AEM.00140-20. Print 2020 May 5. Appl Environ Microbiol. 2020. PMID: 32169939 Free PMC article.
-
Every coin has a back side: invasion by Limnohabitans planktonicus promotes the maintenance of species diversity in bacterial communities.PLoS One. 2012;7(12):e51576. doi: 10.1371/journal.pone.0051576. Epub 2012 Dec 12. PLoS One. 2012. PMID: 23251582 Free PMC article.
-
Contrasting effects of singlet oxygen and hydrogen peroxide on bacterial community composition in a humic lake.PLoS One. 2014 Mar 25;9(3):e92518. doi: 10.1371/journal.pone.0092518. eCollection 2014. PLoS One. 2014. PMID: 24667441 Free PMC article.
-
Niche Separation Increases With Genetic Distance Among Bloom-Forming Cyanobacteria.Front Microbiol. 2018 Mar 27;9:438. doi: 10.3389/fmicb.2018.00438. eCollection 2018. Front Microbiol. 2018. PMID: 29636727 Free PMC article.
References
-
- Alonso, C., M. Zeder, C. Piccini, D. Conde, and J. Pernthaler. 2009. Ecophysiological differences of betaproteobacterial populations in two hydrochemically distinct compartments of a subtropical lagoon. Environ. Microbiol. 11:867-876. - PubMed
-
- Bettarel, Y., T. Sime-Ngando, M. Bouvy, R. Arfi, and C. Amblard. 2005. Low consumption of virus-sized particles by heterotrophic nanoflagellates in two lakes of the French Massif Central. Aquat. Microbiol. Ecol. 39:205-209.
-
- Eiler, A., and S. Bertilsson. 2004. Composition of freshwater bacterial communities associated with cyanobacterial blooms in four Swedish lakes. Environ. Microbiol. 6:1228-1243. - PubMed
-
- Fuhrman, J. A. 1999. Marine viruses and their biogeochemical and ecological effects. Nature 399:541-548. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

