Anthracycline cardiomyopathy is mediated by depletion of the cardiac stem cell pool and is rescued by restoration of progenitor cell function
- PMID: 20038740
- PMCID: PMC2810713
- DOI: 10.1161/CIRCULATIONAHA.109.895771
Anthracycline cardiomyopathy is mediated by depletion of the cardiac stem cell pool and is rescued by restoration of progenitor cell function
Abstract
Background: Anthracyclines are the most effective drugs available in the treatment of neoplastic diseases; however, they have profound consequences on the structure and function of the heart, which over time cause a cardiomyopathy that leads to congestive heart failure.
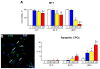
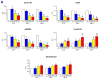
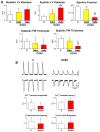
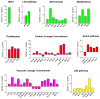
Methods and results: Administration of doxorubicin in rats led to a dilated myopathy, heart failure, and death. To test whether the effects of doxorubicin on cardiac anatomy and function were mediated by alterations in cardiac progenitor cells (CPCs), these cells were exposed to the anthracycline, which increased the formation of reactive oxygen species and caused increases in DNA damage, expression of p53, telomere attrition, and apoptosis. Additionally, doxorubicin resulted in cell-cycle arrest at the G2/M transition, which led to a significant decrease in CPC growth. Doxorubicin elicited multiple molecular adaptations; the massive apoptotic death that occurred in CPCs in the presence of anthracycline imposed on the surviving CPC pool the activation of several pathways aimed at preservation of the primitive state, cell division, lineage differentiation, and repair of damaged DNA. To establish whether delivery of syngeneic progenitor cells opposed the progression of doxorubicin cardiotoxicity, enhanced green fluorescent protein-labeled CPCs were injected in the failing myocardium; this treatment promoted regeneration of cardiomyocytes and vascular structures, which improved ventricular performance and rate of animal survival.
Conclusions: Our results raise the possibility that autologous CPCs can be obtained before antineoplastic drugs are given to cancer patients and subsequently administered to individuals who are particularly sensitive to the cardiotoxicity of these agents for prevention or management of heart failure.
Figures
















References
-
- van Dalen EC, Caron HN, Dickinson HO, Kremer LC. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst Rev. 2008:CD003917. - PubMed
-
- Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis. 2007;49:330–352. - PubMed
-
- Shan K, Lincoff AM, Young JB. Anthracycline-induced cardiotoxicity. Ann Intern Med. 1996;125:47–58. - PubMed
-
- Nithipongvanitch R, Ittarat W, Cole MP, Tangpong J, Clair DK, Oberley TD. Mitochondrial and nuclear p53 localization in cardiomyocytes: redox modulation by doxorubicin (Adriamycin)? Antioxid. Redox Signal. 2007;9:1001–1008. - PubMed
-
- Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 5R01AG017042-09/AG/NIA NIH HHS/United States
- R01 HL111183/HL/NHLBI NIH HHS/United States
- 5R01HL065577-07/HL/NHLBI NIH HHS/United States
- 5R01AG026107-04/AG/NIA NIH HHS/United States
- 5R21HL094894-02/HL/NHLBI NIH HHS/United States
- R01 HL039902/HL/NHLBI NIH HHS/United States
- R01 AG026107/AG/NIA NIH HHS/United States
- R37 HL081737/HL/NHLBI NIH HHS/United States
- 5P01HL092868-02/HL/NHLBI NIH HHS/United States
- 5R37HL081737-05/HL/NHLBI NIH HHS/United States
- R01 AG017042/AG/NIA NIH HHS/United States
- 5R01HL065573-07/HL/NHLBI NIH HHS/United States
- P01 HL092868/HL/NHLBI NIH HHS/United States
- 5R01HL039902-18/HL/NHLBI NIH HHS/United States
- 1R01HL091021-01A2/HL/NHLBI NIH HHS/United States
- R01 HL091021/HL/NHLBI NIH HHS/United States
- R01 HL065577/HL/NHLBI NIH HHS/United States
- 7P01AG023071-05/AG/NIA NIH HHS/United States
- P01 AG023071/AG/NIA NIH HHS/United States
- R21 HL094894/HL/NHLBI NIH HHS/United States
- R01 HL065573/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

