Membrane remodeling during reticulocyte maturation
- PMID: 20038785
- PMCID: PMC2837329
- DOI: 10.1182/blood-2009-08-241182
Membrane remodeling during reticulocyte maturation
Abstract
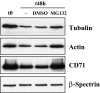
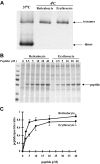
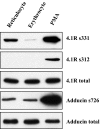
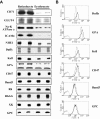
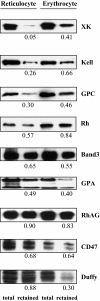
The transition of reticulocytes into erythrocytes is accompanied by extensive changes in the structure and properties of the plasma membrane. These changes include an increase in shear resistance, loss of surface area, and acquisition of a biconcave shape. The processes by which these changes are effected have remained largely undefined. Here we examine how the expression of 30 distinct membrane proteins and their interactions change during murine reticulocyte maturation. We show that tubulin and cytosolic actin are lost, whereas the membrane content of myosin, tropomyosin, intercellular adhesion molecule-4, glucose transporter-4, Na-K-ATPase, sodium/hydrogen exchanger 1, glycophorin A, CD47, Duffy, and Kell is reduced. The degradation of tubulin and actin is, at least in part, through the ubiquitin-proteasome degradation pathway. In regard to the protein-protein interactions, the formation of membrane-associated spectrin tetramers from dimers is unperturbed, whereas the interactions responsible for the formation of the membrane-skeletal junctions are weaker in reticulocytes, as is the attachment of transmembrane proteins to these structures. This weakness, in part, results from the elevated phosphorylation of 4.1R in reticulocytes, which leads to a decrease in shear resistance by reducing its interaction with spectrin and actin. These observations begin to unravel the mechanistic basis of crucial changes accompanying reticulocyte maturation.
Figures

References
-
- Bennett V. The spectrin-actin junction of erythrocyte membrane skeletons. Biochim Biophys Acta. 1989;988(1):107–121. - PubMed
-
- Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev. 2001;81(3):1353–1392. - PubMed
-
- Mohandas N, An X. New insights into function of red cell membrane proteins and their interaction with spectrin-based membrane skeleton. Transfus Clin Biol. 2006;13(1-2):29–30. - PubMed
-
- Manno S, Takakuwa Y, Mohandas N. Modulation of erythrocyte membrane mechanical function by protein 4.1 phosphorylation. J Biol Chem. 2005;280(9):7581–7587. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

