IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury
- PMID: 20038794
- PMCID: PMC2798679
- DOI: 10.1172/JCI38702
IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury
Abstract
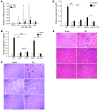
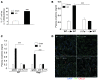
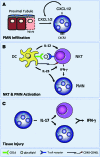
The IL-23/IL-17 and IL-12/IFN-gamma cytokine pathways have a role in chronic autoimmunity, which is considered mainly a dysfunction of adaptive immunity. The extent to which they contribute to innate immunity is, however, unknown. We used a mouse model of acute kidney ischemia-reperfusion injury (IRI) to test the hypothesis that early production of IL-23 and IL-12 following IRI activates downstream IL-17 and IFN-gamma signaling pathways and promotes kidney inflammation. Deficiency in IL-23, IL-17A, or IL-17 receptor (IL-17R) and mAb neutralization of CXCR2, the p19 subunit of IL-23, or IL-17A attenuated neutrophil infiltration in acute kidney IRI in mice. We further demonstrate that IL-17A produced by GR-1+ neutrophils was critical for kidney IRI in mice. Activation of the IL-12/IFN-gamma pathway and NKT cells by administering alpha-galactosylceramide-primed bone marrow-derived DCs increased IFN-gamma production following moderate IRI in WT mice but did not exacerbate injury or enhance IFN-gamma production in either Il17a-/- or Il17r-/- mice, which suggested that IL-17 signaling was proximal to IFN-gamma signaling. This was confirmed by the finding that IFN-gamma administration reversed the protection seen in Il17a-/- mice subjected to IRI, whereas IL-17A failed to reverse protection in Ifng-/- mice. These results demonstrate that the innate immune component of kidney IRI requires dual activation of the IL-12/IFN-gamma and IL-23/IL-17 signaling pathways and that neutrophil production of IL-17A is upstream of IL-12/IFN-gamma. These mechanisms might contribute to reperfusion injury in other organs.
Figures


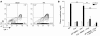

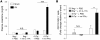
Similar articles
-
Endogenous IL-33 Contributes to Kidney Ischemia-Reperfusion Injury as an Alarmin.J Am Soc Nephrol. 2018 Apr;29(4):1272-1288. doi: 10.1681/ASN.2017060650. Epub 2018 Feb 7. J Am Soc Nephrol. 2018. PMID: 29436517 Free PMC article.
-
NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia-reperfusion injury.J Immunol. 2007 May 1;178(9):5899-911. doi: 10.4049/jimmunol.178.9.5899. J Immunol. 2007. PMID: 17442974
-
HMGB1-TLR4-IL-23-IL-17A axis accelerates renal ischemia-reperfusion injury via the recruitment and migration of neutrophils.Int Immunopharmacol. 2021 May;94:107433. doi: 10.1016/j.intimp.2021.107433. Epub 2021 Feb 13. Int Immunopharmacol. 2021. PMID: 33592404
-
Inflammation in acute kidney injury.Nephron Exp Nephrol. 2008;109(4):e102-7. doi: 10.1159/000142934. Epub 2008 Sep 18. Nephron Exp Nephrol. 2008. PMID: 18802372 Free PMC article. Review.
-
Neutrophils: a cornerstone of liver ischemia and reperfusion injury.Lab Invest. 2018 Jan;98(1):51-62. doi: 10.1038/labinvest.2017.90. Epub 2017 Sep 18. Lab Invest. 2018. PMID: 28920945 Review.
Cited by
-
Dendritic cell sphingosine 1-phosphate receptor-3 regulates Th1-Th2 polarity in kidney ischemia-reperfusion injury.J Immunol. 2012 Sep 1;189(5):2584-96. doi: 10.4049/jimmunol.1200999. Epub 2012 Aug 1. J Immunol. 2012. PMID: 22855711 Free PMC article.
-
Netrin-1 regulates colon-kidney cross talk through suppression of IL-6 function in a mouse model of DSS-colitis.Am J Physiol Renal Physiol. 2013 May 1;304(9):F1187-97. doi: 10.1152/ajprenal.00702.2012. Epub 2013 Feb 27. Am J Physiol Renal Physiol. 2013. PMID: 23445618 Free PMC article.
-
Advances in the study of B cells in renal ischemia-reperfusion injury.Front Immunol. 2023 Nov 1;14:1216094. doi: 10.3389/fimmu.2023.1216094. eCollection 2023. Front Immunol. 2023. PMID: 38022595 Free PMC article. Review.
-
Cytokine control of inflammation and repair in the pathology of multiple sclerosis.Yale J Biol Med. 2012 Dec;85(4):447-68. Epub 2012 Dec 13. Yale J Biol Med. 2012. PMID: 23239947 Free PMC article. Review.
-
MiR-181d-5p Targets KLF6 to Improve Ischemia/Reperfusion-Induced AKI Through Effects on Renal Function, Apoptosis, and Inflammation.Front Physiol. 2020 May 27;11:510. doi: 10.3389/fphys.2020.00510. eCollection 2020. Front Physiol. 2020. PMID: 32581828 Free PMC article.
References
-
- Sun J, et al. TLR ligands can activate dendritic cells to provide a MyD88-dependent negative signal for Th2 cell development. J Immunol. 2005;174(2):742–751. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

