Cardiac fibroblasts are essential for the adaptive response of the murine heart to pressure overload
- PMID: 20038803
- PMCID: PMC2798693
- DOI: 10.1172/JCI40295
Cardiac fibroblasts are essential for the adaptive response of the murine heart to pressure overload
Abstract
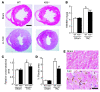
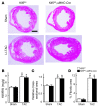
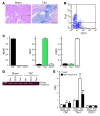
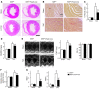
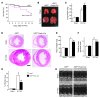
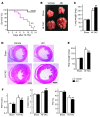
Fibroblasts, which are the most numerous cell type in the heart, interact with cardiomyocytes in vitro and affect their function; however, they are considered to play a secondary role in cardiac hypertrophy and failure. Here we have shown that cardiac fibroblasts are essential for the protective and hypertrophic myocardial responses to pressure overload in vivo in mice. Haploinsufficiency of the transcription factor-encoding gene Krüppel-like factor 5 (Klf5) suppressed cardiac fibrosis and hypertrophy elicited by moderate-intensity pressure overload, whereas cardiomyocyte-specific Klf5 deletion did not alter the hypertrophic responses. By contrast, cardiac fibroblast-specific Klf5 deletion ameliorated cardiac hypertrophy and fibrosis, indicating that KLF5 in fibroblasts is important for the response to pressure overload and that cardiac fibroblasts are required for cardiomyocyte hypertrophy. High-intensity pressure overload caused severe heart failure and early death in mice with Klf5-null fibroblasts. KLF5 transactivated Igf1 in cardiac fibroblasts, and IGF-1 subsequently acted in a paracrine fashion to induce hypertrophic responses in cardiomyocytes. Igf1 induction was essential for cardioprotective responses, as administration of a peptide inhibitor of IGF-1 severely exacerbated heart failure induced by high-intensity pressure overload. Thus, cardiac fibroblasts play a pivotal role in the myocardial adaptive response to pressure overload, and this role is partly controlled by KLF5. Modulation of cardiac fibroblast function may provide a novel strategy for treating heart failure, with KLF5 serving as an attractive target.
Figures
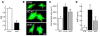

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

