Sorafenib inhibits ERK1/2 and MCL-1(L) phosphorylation levels resulting in caspase-independent cell death in malignant pleural mesothelioma
- PMID: 20038816
- PMCID: PMC3052759
- DOI: 10.4161/cbt.8.24.10824
Sorafenib inhibits ERK1/2 and MCL-1(L) phosphorylation levels resulting in caspase-independent cell death in malignant pleural mesothelioma
Abstract
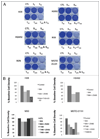
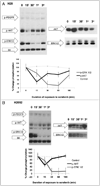
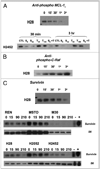
Malignant pleural mesothelioma (MPM) is an aggressive, rapidly progressive malignancy without effective therapy. We evaluate sorafenib efficacy and impact on the cellular pro-survival machinery in vitro, efficacy of sorafenib as monotherapy and in combination with the naturally occurring death receptor agonist, TRAIL using human MPM cell lines, MSTO-211H, M30, REN, H28, H2052 and H2452. In vitro studies of the six MPM lines demonstrated single agent sensitivity to the multikinase inhibitor sorafenib and resistance to TRAIL. H28 and H2452 demonstrated augmented apoptosis with the addition of TRAIL to sorafenib in vitro. Treated cell lines demonstrated sorafenib-induced rapid dephosphorylation of AKT followed shortly by near complete dephosphorylation of the constitutively phosphorylated ERK1/2. Sorafenib therapy also decreased phosphorylation of B-Raf and mTOR in several cell lines. Within 3 h of sorafenib treatment, a number of known pro-survival molecules were dephosphorylated and/or downregulated in expression including MCL-1(L), c-FLIP(L), survivin and cIAP(1). These changes and eventual cell death did not elicit significant caspase-3 activation or PARP cleavage and pretreatment with the pan-caspase inhibitor, Z-VAD-FMK, did not block sorafenib efficacy but did block the effect of TRAIL monotherapy. Pre-treatment with Z-VAD-FMK did not block the synergistic effect of TRAIL and sorafenib in H28. In summary, single agent treatment with sorafenib results in widespread inhibition of the pro-survival machinery in vitro leading to cell death via a primarily caspase-independent mechanism. Combining sorafenib therapy with TRAIL, may be useful in order to provide a more efficient death signal and this synergistic effect appears to be caspase-independent. Pilot in vivo data demonstrates promising evidence of therapeutic efficacy in human tumor bearing xenograft nu/nu mice. We document single agent activity of sorafenib against MPM, unravel novel effects of sorafenib on anti-apoptotic signaling mediators, and suggest the combination of sorafenib plus TRAIL as possible therapy for clinical testing in MPM.
Figures

References
-
- Montanaro F, Rosato R, Gangemi M, et al. Survival of pleural malignant mesothelioma in Italy: a population-based study. Int J Cancer. 2009;124:201–207. - PubMed
-
- Kazan-Allen L. Asbestos and mesothelioma: worldwide trends. Lung Cancer. 2005;49:3–8. - PubMed
-
- Rippo MR, Moretti S, Vescovi S, et al. FLIP overexpression inhibits death receptor-induced apoptosis in malignant mesothelial cells. Oncogene. 2004;23:7753–7760. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
