The beneficial effects of C-Peptide on diabetic polyneuropathy
- PMID: 20039008
- PMCID: PMC2827271
- DOI: 10.1900/RDS.2009.6.187
The beneficial effects of C-Peptide on diabetic polyneuropathy
Abstract
Diabetic polyneuropathy (DPN) is a common complication in diabetes. At present, there is no adequate treatment, and DPN is often debilitating for patients. It is a heterogeneous disorder and differs in type 1 and type 2 diabetes. An important underlying factor in type 1 DPN is insulin deficiency. Proinsulin C-peptide is a critical element in the cascade of events. In this review, we describe the physiological role of C-peptide and how it provides an insulin-like signaling function. Such effects translate into beneficial outcomes in early metabolic perturbations of neural Na+/K+-ATPase and nitric oxide (NO) with subsequent preventive effects on early nerve dysfunction. Further corrective consequences resulting from this signaling cascade have beneficial effects on gene regulation of early gene responses, neurotrophic factors, their receptors, and the insulin receptor itself. This may lead to preventive and corrective results to nerve fiber degeneration and loss, as well as, promotion of nerve fiber regeneration with respect to sensory somatic fibers and small nociceptive nerve fibers. A characteristic abnormality of type 1 DPN is nodal and paranodal degeneration with severe consequences for myelinated fiber function. This review deals in detail with the underlying insulin-deficiency-related molecular changes and their correction by C-peptide. Based on these observations, it is evident that continuous maintenance of insulin-like actions by C-peptide is needed in peripheral nerve to minimize the sequences of metabolic and molecular abnormalities, thereby ameliorating neuropathic complications. There is now ample evidence demonstrating that C-peptide replacement in type 1 diabetes promotes insulin action and signaling activities in a more enhanced, prolonged, and continuous fashion than does insulin alone. It is therefore necessary to replace C-peptide to physiological levels in diabetic patients. This will have substantial beneficial effects on type 1 DPN.
Figures
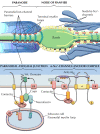
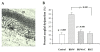
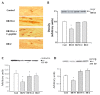
Similar articles
-
Type 1 diabetic neuropathy and C-peptide.Exp Diabesity Res. 2004 Jan-Mar;5(1):65-77. doi: 10.1080/15438600490424541. Exp Diabesity Res. 2004. PMID: 15198372 Free PMC article. Review.
-
C-peptide and diabetic neuropathy.Expert Opin Investig Drugs. 2003 Sep;12(9):1471-88. doi: 10.1517/13543784.12.9.1471. Expert Opin Investig Drugs. 2003. PMID: 12943492 Review.
-
The effects of C-peptide on type 1 diabetic polyneuropathies and encephalopathy in the BB/Wor-rat.Exp Diabetes Res. 2008;2008:230458. doi: 10.1155/2008/230458. Exp Diabetes Res. 2008. PMID: 18437223 Free PMC article. Review.
-
Is C-peptide replacement the missing link for successful treatment of neurological complications in type 1 diabetes?Curr Drug Targets. 2008 Jan;9(1):37-46. doi: 10.2174/138945008783431745. Curr Drug Targets. 2008. PMID: 18220711 Review.
-
C-peptide prevents and improves chronic Type I diabetic polyneuropathy in the BB/Wor rat.Diabetologia. 2001 Jul;44(7):889-97. doi: 10.1007/s001250100570. Diabetologia. 2001. PMID: 11508275
Cited by
-
Generation of a Beta-Cell Transplant Animal Model of Diabetes Using CRISPR Technology.Adv Exp Med Biol. 2023;1409:145-159. doi: 10.1007/5584_2022_746. Adv Exp Med Biol. 2023. PMID: 36289162
-
C-peptide is independent associated with diabetic peripheral neuropathy: a community-based study.Diabetol Metab Syndr. 2017 Feb 13;9:12. doi: 10.1186/s13098-017-0208-2. eCollection 2017. Diabetol Metab Syndr. 2017. PMID: 28228847 Free PMC article.
-
Serum Phosphorylated Neurofilament-Heavy Chain, a Potential Biomarker, is Associated With Peripheral Neuropathy in Patients With Type 2 Diabetes.Medicine (Baltimore). 2015 Nov;94(44):e1908. doi: 10.1097/MD.0000000000001908. Medicine (Baltimore). 2015. PMID: 26554790 Free PMC article.
-
Lentivirus Mediated Pancreatic Beta-Cell-Specific Insulin Gene Therapy for STZ-Induced Diabetes.Mol Ther. 2021 Jan 6;29(1):149-161. doi: 10.1016/j.ymthe.2020.10.025. Epub 2020 Oct 31. Mol Ther. 2021. PMID: 33130311 Free PMC article.
-
Sequential abnormalities in type 1 diabetic encephalopathy and the effects of C-Peptide.Rev Diabet Stud. 2009 Fall;6(3):211-22. doi: 10.1900/RDS.2009.6.211. Epub 2009 Nov 10. Rev Diabet Stud. 2009. PMID: 20039010 Free PMC article.
References
-
- Greene DA, Sima AA, Feldman EL, Stevens MJ. Diabetic neuropathy. In: Porte D Jr, Sherwin RS., editors. Ellenberg and Rifkin Diabetes Mellitus. 2nd ed. Appleton and Lange; Stanford: 1997. pp. 1009–1076.
-
- Sima AA, Kamiya H. Diabetic neuropathy differs in type 1 and type 2 diabetes. Ann NY Acad Sci. 2006;1084:235–249. - PubMed
-
- Dyck PJ, Davies JL, Wilson DM, Service FJ, Melton LJ 3rd, O'Brien PC. Risk factors for severity of diabetic polyneuropathy: intensive longitudinal assessment of the Rochester Diabetic Neuropathy Study Cohort. Diabetes Care. 1999;22:1479–1486. - PubMed
-
- Sima AA. Heterogeneity of diabetic neuropathy. Front Biosci. 2008;13:4809–4816. - PubMed
-
- Sima AA, Bril V, Nathaniel V, McEwen TA, Brown M, Lattimer SA, Greene DA. Regeneration and repair of myelinated fibers in sural nerve biopsies from patients with diabetic neuropathy treated with an aldose reductase inhibitor. N Eng J Med. 1988;319:548–555. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
