Enzymatic properties of the ferredoxin-dependent nitrite reductase from Chlamydomonas reinhardtii. Evidence for hydroxylamine as a late intermediate in ammonia production
- PMID: 20039132
- PMCID: PMC2833264
- DOI: 10.1007/s11120-009-9512-5
Enzymatic properties of the ferredoxin-dependent nitrite reductase from Chlamydomonas reinhardtii. Evidence for hydroxylamine as a late intermediate in ammonia production
Abstract
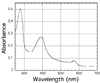
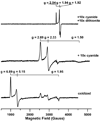
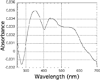
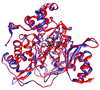
The ferredoxin-dependent nitrite reductase from the green alga Chlamydomonas reinhardtii has been cloned, expressed in Escherichia coli as a His-tagged recombinant protein, and purified to homogeneity. The spectra, kinetic properties and substrate-binding parameters of the C. reinhardtii enzyme are quite similar to those of the ferredoxin-dependent spinach chloroplast nitrite reductase. Computer modeling, based on the published structure of spinach nitrite reductase, predicts that the structure of C. reinhardtii nitrite reductase will be similar to that of the spinach enzyme. Chemical modification studies and the ionic-strength dependence of the enzyme's ability to interact with ferredoxin are consistent with the involvement of arginine and lysine residues on C. reinhardtii nitrite reductase in electrostatically-stabilized binding to ferredoxin. The C. reinhardtii enzyme has been used to demonstrate that hydroxylamine can serve as an electron-accepting substrate for the enzyme and that the product of hydroxylamine reduction is ammonia, providing the first experimental evidence for the hypothesis that hydroxylamine, bound to the enzyme, can serve as a late intermediate during the reduction of nitrite to ammonia catalyzed by the enzyme.
Figures




Similar articles
-
Mechanism of spinach chloroplast ferredoxin-dependent nitrite reductase: spectroscopic evidence for intermediate states.Biochemistry. 2004 Jan 20;43(2):510-7. doi: 10.1021/bi035662q. Biochemistry. 2004. PMID: 14717606
-
The role of tryptophan in the ferredoxin-dependent nitrite reductase of spinach.Photosynth Res. 2007 Oct;94(1):1-12. doi: 10.1007/s11120-007-9198-5. Epub 2007 Jul 5. Photosynth Res. 2007. PMID: 17611813
-
The interaction of spinach nitrite reductase with ferredoxin: a site-directed mutation study.Mol Plant. 2009 May;2(3):407-15. doi: 10.1093/mp/ssn098. Mol Plant. 2009. PMID: 19825625 Free PMC article.
-
Multiple ferredoxin isoforms in Chlamydomonas reinhardtii - their role under stress conditions and biotechnological implications.Eur J Cell Biol. 2010 Dec;89(12):998-1004. doi: 10.1016/j.ejcb.2010.06.018. Epub 2010 Aug 8. Eur J Cell Biol. 2010. PMID: 20696493 Review.
-
Pentahaem cytochrome c nitrite reductase: reaction with hydroxylamine, a potential reaction intermediate and substrate.Biochem Soc Trans. 2002 Aug;30(4):649-53. doi: 10.1042/bst0300649. Biochem Soc Trans. 2002. PMID: 12196156 Review.
Cited by
-
Electrochemical nitrate reduction in acid enables high-efficiency ammonia synthesis and high-voltage pollutes-based fuel cells.Nat Commun. 2023 Dec 5;14(1):8036. doi: 10.1038/s41467-023-43897-6. Nat Commun. 2023. PMID: 38052852 Free PMC article.
-
Role of distal arginine residue in the mechanism of heme nitrite reductases.Chem Sci. 2023 Jun 15;14(29):7875-7886. doi: 10.1039/d3sc01777j. eCollection 2023 Jul 26. Chem Sci. 2023. PMID: 37502318 Free PMC article.
-
The Involvement of hybrid cluster protein 4, HCP4, in Anaerobic Metabolism in Chlamydomonas reinhardtii.PLoS One. 2016 Mar 1;11(3):e0149816. doi: 10.1371/journal.pone.0149816. eCollection 2016. PLoS One. 2016. PMID: 26930496 Free PMC article.
-
Identifying Endogenous Proteins of Perennial Ryegrass (Lolium perenne) with Ex Vivo Antioxidant Activity.Proteomes. 2025 Feb 5;13(1):8. doi: 10.3390/proteomes13010008. Proteomes. 2025. PMID: 39982318 Free PMC article.
-
Redox Regulation of Salt Tolerance in Eutrema salsugineum by Proteomics.Int J Mol Sci. 2023 Sep 25;24(19):14518. doi: 10.3390/ijms241914518. Int J Mol Sci. 2023. PMID: 37833966 Free PMC article.
References
-
- Aparicio PJ, Knaff DB, Malkin R. The role of an iron-sulfur center and siroheme in spinach nitrite reductase. Arch Biochem Biophys. 1975;169:102–107. - PubMed
-
- Bellissimo DB, Privalle LS. Expression of spinach nitrite reductase in Escherichia coli: Site-directed mutagenesis of predicted active site amino acids. Arch Biochem Biophys. 1995;323:155–163. - PubMed
-
- Conway EJ. Microdiffusion Analysis and Volumetric Error. Fourth Edition. London: Crosby, Lookwood and Son, Ltd.; 1957.
-
- Curdt I, Singh BB, Jakoby M, Hachtel W, Bohme H. Identification of amino acid residues of nitrite reductase from Anabaena sp. PCC 7120 involved in ferredoxin binding. Biochem Biophys Acta. 2000;1543:60–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

