Cumulus cell contact during oocyte maturation in mice regulates meiotic spindle positioning and enhances developmental competence
- PMID: 20039198
- PMCID: PMC2826619
- DOI: 10.1007/s10815-009-9376-9
Cumulus cell contact during oocyte maturation in mice regulates meiotic spindle positioning and enhances developmental competence
Abstract
Purpose: To investigate the role of cumulus cell contact during oocyte maturation on meiotic spindle assembly and the acquisition of developmental competence.
Methods: Cumulus oocyte complexes isolated from mouse ovaries subjected to in vitro or in vivo maturation were analyzed by confocal microscopy with respect to oocyte somatic cell contacts and for their ability to develop after parthenogenic activation during embryo culture.
Results: Cell contact is maintained during maturation in vivo, predisposing oocytes to cortical meiotic spindle assembly and developmental competence acquisition. In contrast, oocytes matured in vitro lose cell contact coincident with central meiotic spindle assembly that results in cleavage delays upon egg activation and failure to form blastocysts. Experimental disruption of cell contact by the actin-depolymerizing agent latrunculin B results in the formation of enlarged meiotic spindles with dispersed chromosomes unlike the compact ordering of chromosomes observed on spindles formed after in vivo maturation, suggesting a link between cell contact and the acquisition of developmental competence.
Conclusions: Somatic cell contact optimizes oocyte quality during meiotic maturation by regulating the spatial organization and function of the meiotic spindle through actin-dependent mechanisms that enhance development.
Figures


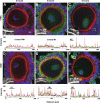
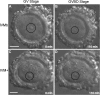
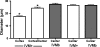
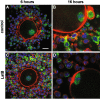
Similar articles
-
Oocyte maturation: gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization.Hum Reprod Update. 2015 Jul-Aug;21(4):427-54. doi: 10.1093/humupd/dmv011. Epub 2015 Mar 4. Hum Reprod Update. 2015. PMID: 25744083 Review.
-
A pre-in vitro maturation medium containing cumulus oocyte complex ligand-receptor signaling molecules maintains meiotic arrest, supports the cumulus oocyte complex and improves oocyte developmental competence.Mol Hum Reprod. 2017 Sep 1;23(9):594-606. doi: 10.1093/molehr/gax032. Mol Hum Reprod. 2017. PMID: 28586460
-
Hormonal regulation of meiotic maturation in the hamster oocyte involves a cytoskeleton-mediated process.Biol Reprod. 1994 Nov;51(5):852-64. doi: 10.1095/biolreprod51.5.852. Biol Reprod. 1994. PMID: 7849187
-
Transporting cumulus complexes using novel meiotic arresting conditions permits maintenance of oocyte developmental competence.J Assist Reprod Genet. 2017 Aug;34(8):1079-1086. doi: 10.1007/s10815-017-0958-7. Epub 2017 Jun 1. J Assist Reprod Genet. 2017. PMID: 28573527 Free PMC article.
-
Control of oocyte meiotic maturation in C. elegans.Semin Cell Dev Biol. 2018 Dec;84:90-99. doi: 10.1016/j.semcdb.2017.12.005. Epub 2017 Dec 26. Semin Cell Dev Biol. 2018. PMID: 29242146 Free PMC article. Review.
Cited by
-
Age, body weight and ovarian function affect oocyte size and morphology in non-PCOS patients undergoing intracytoplasmic sperm injection (ICSI).PLoS One. 2019 Oct 24;14(10):e0222390. doi: 10.1371/journal.pone.0222390. eCollection 2019. PLoS One. 2019. PMID: 31647816 Free PMC article.
-
Mouse oocytes connect with granulosa cells by fusing with cell membranes and form a large complex during follicle development.Biol Reprod. 2018 Sep 1;99(3):527-535. doi: 10.1093/biolre/ioy072. Biol Reprod. 2018. PMID: 29590310 Free PMC article.
-
History, origin, and function of transzonal projections: the bridges of communication between the oocyte and its environment.Anim Reprod. 2018 Aug 16;15(3):215-223. doi: 10.21451/1984-3143-AR2018-0061. Anim Reprod. 2018. PMID: 34178144 Free PMC article.
-
An increase of granulosa cell apoptosis mediates aqueous neem (Azadirachta indica) leaf extract-induced oocyte apoptosis in rat.Int J Appl Basic Med Res. 2013 Jan;3(1):27-36. doi: 10.4103/2229-516X.112238. Int J Appl Basic Med Res. 2013. PMID: 23776837 Free PMC article.
-
Reproductive Ageing: Metabolic contribution to age-related chromosome missegregation in mammalian oocytes.Reproduction. 2024 Jun 28;168(2):e230510. doi: 10.1530/REP-23-0510. Print 2024 Aug 1. Reproduction. 2024. PMID: 38718822 Free PMC article. Review.
References
-
- Albertini DF, Barrett SL. Oocyte-somatic cell communication. Reprod Suppl. 2003;61:49–54. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

