RANKL induces heterogeneous DC-STAMP(lo) and DC-STAMP(hi) osteoclast precursors of which the DC-STAMP(lo) precursors are the master fusogens
- PMID: 20039274
- PMCID: PMC2814997
- DOI: 10.1002/jcp.22012
RANKL induces heterogeneous DC-STAMP(lo) and DC-STAMP(hi) osteoclast precursors of which the DC-STAMP(lo) precursors are the master fusogens
Abstract
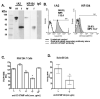
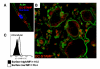
Osteoclasts (OC) are multinucleated bone resorbing cells that form via RANKL-induced fusion of heterogeneous mononuclear OC precursors (OCP). Currently, there are no unique surface markers to distinguish these OCP populations, which are diagnostic for erosive and metabolic bone diseases using culture assays. Thus, we investigated expression of DC-STAMP, a surface receptor required for OCP fusion, during osteoclastogenesis in vitro using a novel monoclonal antibody (1A2). Immunoprecipitation-Western blot analysis of OCP membrane proteins detected 106 kDa dimeric and 53 kDa monomeric DC-STAMP in non-denaturing and denaturing conditions, respectively, with greater sensitivity versus rabbit anti-sera (KR104). 1A2 also detected 99.9% of undifferentiated monocytes as a single population by flow cytometry with a MFI 100-fold over background, while KR104 was not useful in this assay. Functionally, 1A2 inhibited OCP fusion in vitro. RANKL stimulation of OCP induced DC-STAMP(lo) and DC-STAMP(hi) cells, which mature into OC and mononuclear cells respectively as determined by fluorescent microscopy and TRAP assays. Addition of DC-STAMP(hi) cells to purified DC-STAMP(lo) cultures produced larger, more nucleated OC vs. pure DC-STAMP(lo) cultures. RT-qPCR analysis of these two populations showed that OC markers (Trap and Oc-stamp) and fusogenic gene expression (Cd9 and Cd47), were significantly increased in DC-STAMP(lo) vs. DC-STAMP(hi) cells. Collectively, these results demonstrate that DC-STAMP is expressed on OCP as a dimer, which is efficiently detected by 1A2 via flow cytometry. RANKL induces osteoclastogenesis by stimulating DC-STAMP internalization in some OCP, and these DC-STAMP(lo) cells display the "master fusogen" phenotype. In contrast, DC-STAMP(hi) OCP can only act as mononuclear donors.
Keywords: Cell Fusion; Dendritic Cell-Specific Transmembrane Protein (DC-STAMP); Osteoclast Precursors (OCP).
J. Cell. Physiol. 223: 76-83, 2010. (c) 2009 Wiley-Liss, Inc.
Figures



Similar articles
-
Osteoclast stimulatory transmembrane protein (OC-STAMP), a novel protein induced by RANKL that promotes osteoclast differentiation.J Cell Physiol. 2008 May;215(2):497-505. doi: 10.1002/jcp.21331. J Cell Physiol. 2008. PMID: 18064667 Free PMC article.
-
DC-STAMP Is an Osteoclast Fusogen Engaged in Periodontal Bone Resorption.J Dent Res. 2017 Jun;96(6):685-693. doi: 10.1177/0022034517690490. Epub 2017 Feb 15. J Dent Res. 2017. PMID: 28199142 Free PMC article.
-
OC-STAMP promotes osteoclast fusion for pathogenic bone resorption in periodontitis via up-regulation of permissive fusogen CD9.FASEB J. 2018 Jul;32(7):4016-4030. doi: 10.1096/fj.201701424R. Epub 2018 Mar 13. FASEB J. 2018. PMID: 29533736 Free PMC article.
-
Regulators of osteoclast differentiation and cell-cell fusion.Keio J Med. 2011;60(4):101-5. doi: 10.2302/kjm.60.101. Keio J Med. 2011. PMID: 22200633 Review.
-
The dendritic cell-specific transmembrane protein DC-STAMP is essential for osteoclast fusion and osteoclast bone-resorbing activity.Mod Rheumatol. 2006;16(6):341-2. doi: 10.1007/s10165-006-0524-0. Epub 2006 Dec 20. Mod Rheumatol. 2006. PMID: 17164993 Review.
Cited by
-
The Dark Side of Cell Fusion.Int J Mol Sci. 2016 Apr 28;17(5):638. doi: 10.3390/ijms17050638. Int J Mol Sci. 2016. PMID: 27136533 Free PMC article. Review.
-
Osteoclast Fusion: Time-Lapse Reveals Involvement of CD47 and Syncytin-1 at Different Stages of Nuclearity.J Cell Physiol. 2017 Jun;232(6):1396-1403. doi: 10.1002/jcp.25633. Epub 2016 Oct 19. J Cell Physiol. 2017. PMID: 27714815 Free PMC article.
-
Learning from Monocyte-Macrophage Fusion and Multinucleation: Potential Therapeutic Targets for Osteoporosis and Rheumatoid Arthritis.Int J Mol Sci. 2020 Aug 20;21(17):6001. doi: 10.3390/ijms21176001. Int J Mol Sci. 2020. PMID: 32825443 Free PMC article. Review.
-
STATs and macrophage fusion.JAKSTAT. 2013 Jul 1;2(3):e24777. doi: 10.4161/jkst.24777. Epub 2013 Apr 23. JAKSTAT. 2013. PMID: 24069561 Free PMC article. Review.
-
The Implant-Induced Foreign Body Response Is Limited by CD13-Dependent Regulation of Ubiquitination of Fusogenic Proteins.J Immunol. 2024 Feb 15;212(4):663-676. doi: 10.4049/jimmunol.2300688. J Immunol. 2024. PMID: 38149920 Free PMC article.
References
-
- Anandarajah AP, EM Schwarz, Totterman S, Monu J, Feng CY, Shao T, Haas-Smith SA, Ritchlin CT. The effect of etanercept on osteoclast precursor frequency and enhancing bone marrow oedema in patients with psoriatic arthritis. Ann Rheum Dis. 2008;67(3):296–301. - PubMed
-
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–342. - PubMed
-
- Chen EH, Grote E, Mohler W, Vignery A. Cell-cell fusion. FEBS Lett. 2007;581(11):2181–2193. - PubMed
-
- Cohen SB, Dore RK, Lane NE, Ory PA, Peterfy CG, Sharp JT, van der Heijde D, Zhou L, Tsuji W, Newmark R. Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum. 2008;58(5):1299–1309. - PubMed
-
- Dalbeth N, Smith T, Nicolson B, Clark B, Callon K, Naot D, Haskard DO, McQueen FM, Reid IR, Cornish J. Enhanced osteoclastogenesis in patients with tophaceous gout: urate crystals promote osteoclast development through interactions with stromal cells. Arthritis Rheum. 2008;58(6):1854–1865. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

