S1P3 confers differential S1P-induced migration by autoreactive and non-autoreactive immature B cells and is required for normal B-cell development
- PMID: 20039302
- PMCID: PMC2924669
- DOI: 10.1002/eji.200939858
S1P3 confers differential S1P-induced migration by autoreactive and non-autoreactive immature B cells and is required for normal B-cell development
Abstract
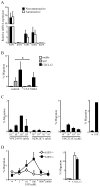
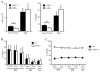
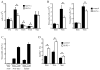
During B-cell development, immature B-cell fate is determined by whether the BCR is engaged in the bone marrow. Immature B cells that are non-autoreactive continue maturation and emigrate from the marrow, whereas autoreactive immature B cells remain and are tolerized. However, the microenvironment where these events occur and the chemoattractants responsible for immature B-cell trafficking within and out of the bone marrow remain largely undefined. Sphingosine 1-phosphate (S1P) is a chemoattractant that directs lymphocyte trafficking and thymocyte egress and in this study we investigated whether S1P contributes to B-cell development, egress and positioning within the bone marrow. Our findings show that immature B cells are chemotactic toward S1P but that this response is dependent on Ag receptor specificity: non-autoreactive, but not autoreactive, immature B cells migrate toward S1P and are shown to require S1P3 receptor for this response. Despite this response, S1P3 is shown not to facilitate immature B-cell egress but is required for normal B-cell development including the positioning of transitional B cells within bone marrow sinusoids. These data indicate that S1P3 signaling directs immature B cells to a bone marrow microenvironment important for both tolerance induction and maturation.
Conflict of interest statement
The authors declare no financial or commercial conflict of interest.
Figures

Similar articles
-
A role for S1P and S1P1 in immature-B cell egress from mouse bone marrow.PLoS One. 2010 Feb 18;5(2):e9277. doi: 10.1371/journal.pone.0009277. PLoS One. 2010. PMID: 20174580 Free PMC article.
-
The origin and maturity of dendritic cells determine the pattern of sphingosine 1-phosphate receptors expressed and required for efficient migration.J Immunol. 2010 Oct 1;185(7):4072-81. doi: 10.4049/jimmunol.1000568. Epub 2010 Sep 8. J Immunol. 2010. PMID: 20826749
-
Selective coupling of the S1P3 receptor subtype to S1P-mediated RhoA activation and cardioprotection.J Mol Cell Cardiol. 2017 Feb;103:1-10. doi: 10.1016/j.yjmcc.2016.12.008. Epub 2016 Dec 23. J Mol Cell Cardiol. 2017. PMID: 28017639 Free PMC article.
-
The influence of sphingosine-1-phosphate receptor signaling on lymphocyte trafficking: how a bioactive lipid mediator grew up from an "immature" vascular maturation factor to a "mature" mediator of lymphocyte behavior and function.Immunol Res. 2009;43(1-3):187-97. doi: 10.1007/s12026-008-8066-5. Immunol Res. 2009. PMID: 18854957 Review.
-
The role of the lysophospholipid sphingosine 1-phosphate in immune cell biology.Arch Immunol Ther Exp (Warsz). 2006 Jul-Aug;54(4):239-51. doi: 10.1007/s00005-006-0028-9. Epub 2006 Jul 10. Arch Immunol Ther Exp (Warsz). 2006. PMID: 16830220 Review.
Cited by
-
S1P1 receptor directs the release of immature B cells from bone marrow into blood.J Exp Med. 2010 May 10;207(5):1113-24. doi: 10.1084/jem.20092210. Epub 2010 Apr 19. J Exp Med. 2010. PMID: 20404103 Free PMC article.
-
Fingolimod is a potential novel therapy for multiple sclerosis.Nat Rev Neurol. 2010 Jul;6(7):373-82. doi: 10.1038/nrneurol.2010.76. Epub 2010 Jun 15. Nat Rev Neurol. 2010. PMID: 20551946 Review.
-
B-cell intrinsic and extrinsic signals that regulate central tolerance of mouse and human B cells.Immunol Rev. 2022 May;307(1):12-26. doi: 10.1111/imr.13062. Epub 2022 Jan 8. Immunol Rev. 2022. PMID: 34997597 Free PMC article. Review.
-
CXCR4 and a cell-extrinsic mechanism control immature B lymphocyte egress from bone marrow.J Exp Med. 2014 Dec 15;211(13):2567-81. doi: 10.1084/jem.20140457. Epub 2014 Nov 17. J Exp Med. 2014. PMID: 25403444 Free PMC article.
-
Expression of functional sphingosine-1 phosphate receptor-1 is reduced by B cell receptor signaling and increased by inhibition of PI3 kinase δ but not SYK or BTK in chronic lymphocytic leukemia cells.J Immunol. 2015 Mar 1;194(5):2439-46. doi: 10.4049/jimmunol.1402304. Epub 2015 Jan 28. J Immunol. 2015. PMID: 25632006 Free PMC article.
References
-
- Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–758. - PubMed
-
- Meffre E, Casellas R, Nussenzweig MC. Antibody regulation of B cell development. Nat Immunol. 2000;1:379–385. - PubMed
-
- Monroe JG, Dorshkind K. Fate decisions regulating bone marrow and peripheral B lymphocyte development. Adv Immunol. 2007;95:1–50. - PubMed
-
- Shlomchik MJ. Sites and stages of autoreactive B cell activation and regulation. Immunity. 2008;28:18–28. - PubMed
-
- Pelanda R, Torres RM. Receptor editing for better or for worse. Curr Opin Immunol. 2006;18:184–190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

