Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial
- PMID: 20039413
- PMCID: PMC4548300
- DOI: 10.1002/art.27233
Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial
Abstract
Objective: B cells are likely to contribute to the pathogenesis of systemic lupus erythematosus (SLE), and rituximab induces depletion of B cells. The Exploratory Phase II/III SLE Evaluation of Rituximab (EXPLORER) trial tested the efficacy and safety of rituximab versus placebo in patients with moderately-to-severely active extrarenal SLE.
Methods: Patients entered with >or=1 British Isles Lupus Assessment Group (BILAG) A score or >or=2 BILAG B scores despite background immunosuppressant therapy, which was continued during the trial. Prednisone was added and subsequently tapered. Patients were randomized at a ratio of 2:1 to receive rituximab (1,000 mg) or placebo on days 1, 15, 168, and 182.
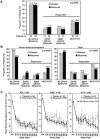
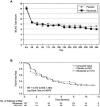
Results: In the intent-to-treat analysis of 257 patients, background treatment was evenly distributed among azathioprine, mycophenolate mofetil, and methotrexate. Fifty-three percent of the patients had >or=1 BILAG A score at entry, and 57% of the patients were categorized as being steroid dependent. No differences were observed between placebo and rituximab in the primary and secondary efficacy end points, including the BILAG-defined response, in terms of both area under the curve and landmark analyses. A beneficial effect of rituximab on the primary end point was observed in the African American and Hispanic subgroups. Safety and tolerability were similar in patients receiving placebo and those receiving rituximab.
Conclusion: The EXPLORER trial enrolled patients with moderately-to-severely active SLE and used aggressive background treatment and sensitive cutoffs for nonresponse. No differences were noted between placebo and rituximab in the primary and secondary end points. Further evaluation of patient subsets, biomarkers, and exploratory outcome models may improve the design of future SLE clinical trials.
Trial registration: ClinicalTrials.gov NCT00137969.
Figures


Comment in
-
Unanswered questions in evaluating rituximab efficacy: comment on the article by Merrill et al.Arthritis Rheum. 2010 Aug;62(8):2566. doi: 10.1002/art.27557. Arthritis Rheum. 2010. PMID: 20496370 No abstract available.
References
-
- Gill JM, Quisel AM, Rocca PV, Walters DT. Diagnosis of systemic lupus erythematosus. Am Fam Physician. 2003;68:2179–86. - PubMed
-
- Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358:929–39. - PubMed
-
- Stoll T, Seifert B, Isenberg DA. SLICC/ACR damage index is valid, and renal and pulmonary organ scores are predictors of severe outcome in patients with systemic lupus erythematosus. Br J Rheumatol. 1996;35:248–54. - PubMed
-
- Dooley MA, Hogan S, Jennette C, Falk R, for the Glomerular Disease Collaborative Network Cyclophosphamide therapy for lupus nephritis: poor renal survival in black Americans. Kidney Int. 1997;51:1188–95. - PubMed
-
- Alarcon GS, Roseman JM, McGwin G, Jr, Uribe A, Bastian HM, Fessler BJ, et al. for the LUMINA study group Systemic lupus erythematosus in three ethnic groups. XX. Damage as a predictor of further damage. Rheumatology (Oxford) 2004;43:202–5. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

